Long Non-Coding RNA DUXAP8 Enhances Renal Cell Carcinoma Progression via Downregulating miR-126
- PMID: 30317248
- PMCID: PMC6198709
- DOI: 10.12659/MSM.910054
Long Non-Coding RNA DUXAP8 Enhances Renal Cell Carcinoma Progression via Downregulating miR-126
Abstract
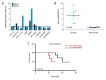
BACKGROUND Renal cell carcinoma (RCC) is one of the common malignant tumors in the urinary system, which endangers human health for a long time. The past decade, the molecular biology of renal cell carcinoma has made considerable progress, so that we have a more profound understanding of renal cell carcinoma. Molecular biological mechanism of renal cell carcinoma remains to be explored. Evidence indicates that long non-coding RNAs (lncRNAs) may be important players in human cancer progression, including RCC. In this study, we found that a newly discovered pseudogene-derived lncRNA named DUXAP8, a 2107-bp RNA, was remarkably upregulated in RCC. MATERIAL AND METHODS Expression of lncRNA DUXAP8 was determined by a qRT-PCR assay in RCC tissues. The proliferation and invasion of RCC cell were measured by a cell proliferation assay and a Transwell invasion assay. Expression of miR-126 was detected by real-time PCR. Interactions between lncRNA DUXAP8 and miR-126 were measured by a luciferase reporter assay and an RNA-pull down assay. In vivo experiments were used to detect tumor formation. RESULTS Together, our study not only identifies lncRNA DUXAP8 as a negative regulator of renal cancer with potential clinical value, but also reveals a regulatory mechanism by long non-coding RNAs to control tumor development. CONCLUSIONS Results from this study provide evidence that lncRNA DUXAP8 enhances renal cell carcinoma progression via downregulating of miR-126, which offers a new approach for the treatment of RCC.
Conflict of interest statement
None.
Figures




References
-
- Meyer AR, Allaf ME, Rowe SP, Gorin MA. The role of molecular imaging in the characterization of renal masses. Curr Opin Urol. 2018;28(2):159–65. - PubMed
-
- Sun M, De Velasco G, Brastianos PK, et al. The development of brain metastases in patients with renal cell carcinoma: Epidemiologic trends, survival, and clinical risk factors using a population-based cohort. Eur Urol Focus. :2018. [Epub ahead of print] - PubMed
-
- Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–89. - PubMed
-
- Mouallem NE, Smith SC, Paul AK. Sarcomatoid renal cell carcinoma: Biology and treatment advances. Urol Oncol. 2018;36:265–71. - PubMed
-
- Macoska JA, Trybus TM, Benson PD, et al. Evidence for three tumor suppressor gene loci on chromosome 8p in human prostate cancer. Cancer Res. 1995;55:5390–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

