Metformin Targets Mitochondrial Electron Transport to Reduce Air-Pollution-Induced Thrombosis
- PMID: 30318339
- PMCID: PMC6365216
- DOI: 10.1016/j.cmet.2018.09.019
Metformin Targets Mitochondrial Electron Transport to Reduce Air-Pollution-Induced Thrombosis
Erratum in
-
Metformin Targets Mitochondrial Electron Transport to Reduce Air-Pollution-Induced Thrombosis.Cell Metab. 2019 Feb 5;29(2):503. doi: 10.1016/j.cmet.2018.12.001. Cell Metab. 2019. PMID: 30726761 Free PMC article. No abstract available.
Abstract
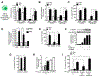
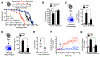
Urban particulate matter air pollution induces the release of pro-inflammatory cytokines including interleukin-6 (IL-6) from alveolar macrophages, resulting in an increase in thrombosis. Here, we report that metformin provides protection in this murine model. Treatment of mice with metformin or exposure of murine or human alveolar macrophages to metformin prevented the particulate matter-induced generation of complex III mitochondrial reactive oxygen species, which were necessary for the opening of calcium release-activated channels (CRAC) and release of IL-6. Targeted genetic deletion of electron transport or CRAC channels in alveolar macrophages in mice prevented particulate matter-induced acceleration of arterial thrombosis. These findings suggest metformin as a potential therapy to prevent some of the premature deaths attributable to air pollution exposure worldwide.
Keywords: air pollution; calcium channels; electron transport chain; mitochondria; proteostasis; reactive oxygen species; signaling; thrombosis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



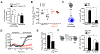

References
-
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, and Leverve XM (2006). The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. Journal of bioenergetics and biomembranes 38, 33–42. - PubMed
-
- Bharat A, Bhorade SM, Morales-Nebreda L, McQuattie-Pimentel AC, Soberanes S, Ridge K, DeCamp MM, Mestan KK, Perlman H, Budinger GR, et al. (2016). Flow Cytometry Reveals Similarities Between Lung Macrophages in Humans and Mice. American journal of respiratory cell and molecular biology 54, 147–149. - PMC - PubMed
-
- Brand MD, Goncalves RLS, Orr AL, Vargas L, Gerencser AA, Jensen MB, Wang YT, Melov S, Turk CN, Matzen JT, et al. (2016). Suppressors of Superoxide-H(2)O(2) Production at Site I(Q) of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell metabolism 24, 582–592. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 CX001777/CX/CSRD VA/United States
- R01 ES013995/ES/NIEHS NIH HHS/United States
- R37 HL048129/HL/NHLBI NIH HHS/United States
- K08 HL125940/HL/NHLBI NIH HHS/United States
- R01 HL048129/HL/NHLBI NIH HHS/United States
- K01 AR066579/AR/NIAMS NIH HHS/United States
- R21 ES025644/ES/NIEHS NIH HHS/United States
- R03 AR061593/AR/NIAMS NIH HHS/United States
- R01 GM114210/GM/NIGMS NIH HHS/United States
- I01 BX000201/BX/BLRD VA/United States
- K08 HL128867/HL/NHLBI NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 AR064546/AR/NIAMS NIH HHS/United States
- R01 NS057499/NS/NINDS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- R01 HL128194/HL/NHLBI NIH HHS/United States
- P30 ES027792/ES/NIEHS NIH HHS/United States
- U01 ES026718/ES/NIEHS NIH HHS/United States
- R01 HL124664/HL/NHLBI NIH HHS/United States
- R01 HL134375/HL/NHLBI NIH HHS/United States
- R01 HL085534/HL/NHLBI NIH HHS/United States
- R01 HL079190/HL/NHLBI NIH HHS/United States
- R56 HL135124/HL/NHLBI NIH HHS/United States
- R35 CA197532/CA/NCI NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

