Neonatal Tbr1 Dosage Controls Cortical Layer 6 Connectivity
- PMID: 30318412
- PMCID: PMC6250594
- DOI: 10.1016/j.neuron.2018.09.027
Neonatal Tbr1 Dosage Controls Cortical Layer 6 Connectivity
Abstract
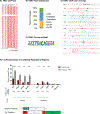
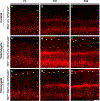
An understanding of how heterozygous loss-of-function mutations in autism spectrum disorder (ASD) risk genes, such as TBR1, contribute to ASD remains elusive. Conditional Tbr1 deletion during late mouse gestation in cortical layer 6 neurons (Tbr1layer6 mutants) provides novel insights into its function, including dendritic patterning, synaptogenesis, and cell-intrinsic physiology. These phenotypes occur in heterozygotes, providing insights into mechanisms that may underlie ASD pathophysiology. Restoring expression of Wnt7b largely rescues the synaptic deficit in Tbr1layer6 mutant neurons. Furthermore, Tbr1layer6 heterozygotes have increased anxiety-like behavior, a phenotype seen ASD. Integrating TBR1 chromatin immunoprecipitation sequencing (ChIP-seq) and RNA sequencing (RNA-seq) data from layer 6 neurons and activity of TBR1-bound candidate enhancers provides evidence for how TBR1 regulates layer 6 properties. Moreover, several putative TBR1 targets are ASD risk genes, placing TBR1 in a central position both for ASD risk and for regulating transcriptional circuits that control multiple steps in layer 6 development essential for the assembly of neural circuits.
Keywords: ASD; Tbr1; aggression; anxiety-like behavior; cortical development; development; layer 6; synapses.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
J.L.R.R. is cofounder, stockholder, and currently on the scientific board of
Figures






References
-
- BEDOGNI F, HODGE RD, ELSEN GE, NELSON BR, DAZA RAM, BEYER RP, BAMMLER TK, RUBENSTEIN JLR & HEVNER RF (2010). Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proceedings of the National Academy of Sciences, 107, 13129–13134. https://doi.org/10.1073/pnas.1002285107 - DOI - PMC - PubMed
-
- BUDNIK V & SALINAS PC (2011). Wnt signaling during synaptic development and plasticity. Current Opinion in Neurobiology, 21, 151–159. https://doi.org/10.1016/j.conb.2010.12.002 - DOI - PMC - PubMed
-
- BULFONE A, SMIGA SM, SHIMAMURA K, PETERSON A, PUELLES L & RUBENSTEIN JLR (1995). T-Brain-1: A homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron, 15, 63–78. https://doi.org/10.1016/0896-6273(95)90065-9 - DOI - PubMed
-
- BULFONE A, WANG F, HEVNER R, ANDERSON S, CUTFORTH T, CHEN S, MENESES J, PEDERSEN R, AXEL R & RUBENSTEIN JLR (1998). An Olfactory Sensory Map Develops in the Absence of Normal Projection Neurons or GABAergic Interneurons. Neuron, 21, 1273–1282. https://doi.org/10.1016/S08966273(00)80647-9 - DOI - PubMed
-
- CHEVÉE M, ROBERTSON JDJ, CANNON GH, BROWN SP & GOFF LA (2018). Variation in Activity State, Axonal Projection, and Position Define the Transcriptional Identity of Individual Neocortical Projection Neurons. Cell Reports, 22, 441–455. https://doi.org/10.1016/j.celrep.2017.12.046 - DOI - PMC - PubMed

