A Lysine Acetyltransferase Contributes to the Metabolic Adaptation to Hypoxia in Mycobacterium tuberculosis
- PMID: 30318462
- PMCID: PMC6309504
- DOI: 10.1016/j.chembiol.2018.09.009
A Lysine Acetyltransferase Contributes to the Metabolic Adaptation to Hypoxia in Mycobacterium tuberculosis
Abstract
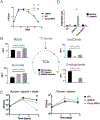
Upon inhibition of respiration, which occurs in hypoxic or nitric oxide-containing host microenvironments, Mycobacterium tuberculosis (Mtb) adopts a non-replicating "quiescent" state and becomes relatively unresponsive to antibiotic treatment. We used comprehensive mutant fitness analysis to identify regulatory and metabolic pathways that are essential for the survival of quiescent Mtb. This genetic study identified a protein acetyltransferase (Mt-Pat/Rv0998) that promoted survival and altered the flux of carbon from oxidative to reductive tricarboxylic acid (TCA) reactions. Reductive TCA requires malate dehydrogenase (MDH) and maintains the redox state of the NAD+/NADH pool. Genetic or chemical inhibition of MDH resulted in rapid cell death in both hypoxic cultures and in murine lung. These phenotypic data, in conjunction with significant structural differences between human and mycobacterial MDH enzymes that could be exploited for drug development, suggest a new strategy for eradicating quiescent bacteria.
Keywords: antibiotic; metabolism; mycobacterium; tuberculosis.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare no competing interests.
Figures
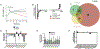
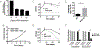



References
-
- Castaño-Cerezo S, Bernal V, Blanco-Catalá J, Iborra JL, and Cánovas M (2011).cAMP-CRP co-ordinates the expression of the protein acetylation pathway with central metabolism in Escherichia coli. Mol. Microbiol 82, 1110–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

