Cumulative Effects of LDL Cholesterol and CRP Levels on Recurrent Stroke and TIA
- PMID: 30318492
- PMCID: PMC6514170
- DOI: 10.5551/jat.45989
Cumulative Effects of LDL Cholesterol and CRP Levels on Recurrent Stroke and TIA
Abstract
Aims: To investigate the relative contribution of on-treatment low-density lipoprotein (LDL) cholesterol and C-reactive protein (CRP) to the risk of recurrent stroke and transient ischemic attack (TIA) in patients with history of ischemic stroke.
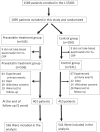
Methods: A total of 1095 patients with non-cardioembolic ischemic stroke were randomized into two groups: control and patients receiving 10 mg of pravastatin per day. After excluding 18 patients who did not have baseline CRP data, the effects of LDL cholesterol and CRP on recurrent stroke and TIA were prospectively assessed in 1077 patients.
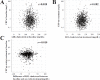
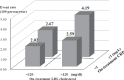
Results: During the follow-up of 4.9±1.4 years, there were 131 recurrent stroke or TIA cases. Patients with ontreatment LDL cholesterol <120 mg/dL showed 29% reduction in recurrent stroke and TIA than those with LDL cholesterol ≥ 120 mg/dL (event rate 2.20 vs. 3.11 per 100 person-years, hazard ratio [HR] 0.71, 95% confidence interval (CI) 0.50-0.99, p=0.048). Patients with CRP <1 mg/L had 32% reduction compared with that of patients with CRP ≥ 1 mg/L (event rate 2.26 vs. 3.40 per 100 person-years; HR 0.68, 95% CI 0.48-0.96, p=0.031). Although LDL cholesterol and CRP levels were not correlated in individual patients, those who achieved both LDL cholesterol <120 mg/dL and CRP <1 mg/L showed 51% reduction compared with that of patients with LDL cholesterol ≥ 120 mg/dL and CRP ≥ 1 mg/L (event rate 2.02 vs. 4.19 per 100 person-years; HR 0.49, 95% CI 0.31-0.79).
Conclusions: The control of both LDL cholesterol and CRP levels appears to be effective for preventing recurrent stroke and TIA in patients with non-cardiogenic ischemic stroke.
Keywords: Cholesterol; Crp; Inflammation; Statin; Stroke prevention.
Conflict of interest statement
KK serves on the speakers' bureau of Takeda Pharmaceutical Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Company, Limited, MSD K.K., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., Shionogi Inc., Pfizer Inc., Bristol-Myers Squibb Company, Bayer Yakuhin, Ltd., received research grant from Grants-in-Aid for Scientific Research (15H04844), Takeda Pharmaceutical Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Company, Limited, MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., AstraZeneca K.K., Kyowa Hakko Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., Eisai Co., Ltd, Pfizer Inc., and Bayer Yakuhin, Ltd. NH serves on the speakers' bureau of Mochida Phamaceutical Co., Ltd. HM received research grant from Daiichi Sankyo Company, Eisai Co., Ltd, Pfizer Inc., Takeda Pharmaceutical Company Limited, Otsuka Inc., Nihon Pharmaceutical, Shionogi, Teijin Pharma, Fuji Film, Nippon Boehringer Ingelheim Co., Ltd., Limited, Nihon Medi-Physics, Bayer Yakuhin, Ltd., MSD K.K., Kyowa Hakko Kirin Co., Ltd., Novartis, Mitsubishi Tanabe Pharma. KM serves on the speakers' bureau of Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin,. SU serves on the speakers' bureau of Boehringer Ingelheim, Bristol-Myers Squibb, and Bayer Yakuhin MM serves on the speakers' bureau of Takeda Pharmaceutical Company Limited, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Company, Limited, MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., Bristol-Myers Squibb Company, Bayer Yakuhin, Ltd., Novartis Pharma K.K., received research grant from Daiichi Sankyo Company, Eisai Co., Ltd, Pfizer Inc., Takeda Pharmaceutical Company Limited, Otsuka Inc., Nihon Pharmaceutical, Nippon Boehringer Ingelheim Co., Ltd., Limited, Nihon Medi-Physics, Bayer Yakuhin, Ltd., MSD K.K., Mitsubishi-Tanabe Pharma, Shionogi IncOther authors had no conflicts of interest.
Figures


References
-
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129 (25 Suppl 2): S1-45 - PubMed
-
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA, 2001; 286: 64-70 - PubMed
-
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med, 2008; 359: 2195-2207 - PubMed
-
- Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M, J-STARS Collaborators A Multicenter, Randomized, Open-label, Parallel-group Study. EBioMedicine, 2015; 2: 1071-1078 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

