Target-based therapeutic matching of phase I trials in patients with metastatic breast cancer in a tertiary referral centre
- PMID: 30318518
- PMCID: PMC6203714
- DOI: 10.1038/s41416-018-0290-8
Target-based therapeutic matching of phase I trials in patients with metastatic breast cancer in a tertiary referral centre
Abstract
Background: Greater understanding of the molecular classification of breast cancer has permitted the development of rational drug design strategies. In a phase I clinical trial setting, molecular profiling with next-generation sequencing of individual tumour samples has been employed to guide treatment.
Methods: We conducted a retrospective evaluation of clinical outcomes of patients with metastatic breast cancer (MBC) treated in phase I clinical trials at our institution to assess the benefit of molecularly matched compared to non-matched treatments.
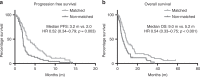
Results: A total of 97 consecutive patients with MBC were enrolled onto ≥1 trial between 2009 and 2015. Fourteen patients participated in multiple trials, and a total of 113 trial encounters were reviewed in this retrospective study. Eighty-three percent of patients with molecular data available were able to participate in trials matched to molecular aberrations. Patients who were treated on matched studies had improved clinical benefit (RR: 1.80, p = 0.005), progression-free (HR: 0.52, p = 0.003) and overall survival (HR: 0.54, p < 0.001). Treatment was well tolerated with low rates of treatment discontinuation for toxicity (8% overall) that did not differ between groups. No toxicity-related deaths were observed.
Conclusions: Molecular profiling for MBC patients in a phase I setting is feasible and aids therapeutic decisions with improved patient outcomes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. - DOI - PubMed
-
- National Cancer Institute. Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events v3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

