Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs
- PMID: 30319321
- PMCID: PMC6181228
- DOI: 10.1002/adfm.201605352
Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs
Abstract
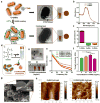
Bioprinting is the most convenient microfabrication method to create biomimetic three-dimensional (3D) cardiac tissue constructs, which can be used to regenerate damaged tissue and provide platforms for drug screening. However, existing bioinks, which are usually composed of polymeric biomaterials, are poorly conductive and delay efficient electrical coupling between adjacent cardiac cells. To solve this problem, we developed a gold nanorod (GNR) incorporated gelatin methacryloyl (GelMA)-based bioink for printing 3D functional cardiac tissue constructs. The GNR concentration was adjusted to create a proper microenvironment for the spreading and organization of cardiac cells. At optimized concentration of GNR, the nanocomposite bioink had a low viscosity, similar to pristine inks, which allowed for the easy integration of cells at high densities. As a result, rapid deposition of cell-laden fibers at a high resolution was possible, while reducing shear stress on the encapsulated cells. In the printed GNR constructs, cardiac cells showed improved cell adhesion and organization when compared to the constructs without GNRs. Furthermore, the incorporated GNRs bridged the electrically resistant pore walls of polymers, improved the cell-to-cell coupling, and promoted synchronized contraction of the bioprinted constructs. Given its advantageous properties, this gold nanocomposite bioink may find wide application in cardiac tissue engineering.
Keywords: Alginate; Bioprinting; Cardiac tissue engineering; Gelatin; Gold nanorods.
Conflict of interest statement
The authors declare no conflict of interests in this work.
Figures




References
-
- Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. Adv. Mater. 2014;26:3124. - PubMed
-
- Blaeser A, Campos DFD, Puster U, Richtering W, Stevens MM, Fischer H. Adv. Healthcare Mater. 2015;5:326. - PubMed
-
- Tamayol A, Najafabadi AH, Aliakbarian B, Arab-Tehrany E, Akbari M, Annabi N, Juncker D, Khademhosseini A. Adv. Healthcare Mater. 2015;4:2146. - PubMed
- Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A. Biomaterials. 2016;106:58. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
