Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models
- PMID: 30319550
- PMCID: PMC6170611
- DOI: 10.3389/fendo.2018.00570
Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models
Abstract
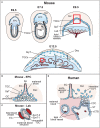
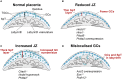
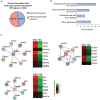
The placenta is the chief regulator of nutrient supply to the growing embryo during gestation. As such, adequate placental function is instrumental for developmental progression throughout intrauterine development. One of the most common complications during pregnancy is insufficient growth of the fetus, a problem termed intrauterine growth restriction (IUGR) that is most frequently rooted in a malfunctional placenta. Together with conventional gene targeting approaches, recent advances in screening mouse mutants for placental defects, combined with the ability to rapidly induce mutations in vitro and in vivo by CRISPR-Cas9 technology, has provided new insights into the contribution of the genome to normal placental development. Most importantly, these data have demonstrated that far more genes are required for normal placentation than previously appreciated. Here, we provide a summary of common types of placental defects in established mouse mutants, which will help us gain a better understanding of the genes impacting on human placentation. Based on a recent mouse mutant screen, we then provide examples on how these data can be mined to identify novel molecular hubs that may be critical for placental development. Given the close association between placental defects and abnormal cardiovascular and brain development, these functional nodes may also shed light onto the etiology of birth defects that co-occur with placental malformations. Taken together, recent insights into the regulation of mouse placental development have opened up new avenues for research that will promote the study of human pregnancy conditions, notably those based on defects in placentation that underlie the most common pregnancy pathologies such as IUGR and pre-eclampsia.
Keywords: DMDD; IUGR; fetal growth restriction; mouse models; placenta; trophoblast.
Figures

References
-
- Vijayaselvi R, Cherian AG. Risk assessment of intrauterine growth restriction. Curr Med Issues (2017) 15:262–6. 10.4103/cmi.cmi_76_17 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

