The Anti-tumoral Properties of Orexin/Hypocretin Hypothalamic Neuropeptides: An Unexpected Therapeutic Role
- PMID: 30319552
- PMCID: PMC6170602
- DOI: 10.3389/fendo.2018.00573
The Anti-tumoral Properties of Orexin/Hypocretin Hypothalamic Neuropeptides: An Unexpected Therapeutic Role
Abstract
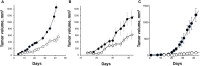
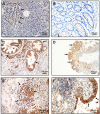
Orexins (OxA and OxB) also termed hypocretins are hypothalamic neuropeptides involved in central nervous system (CNS) to control the sleep/wake process which is mediated by two G protein-coupled receptor subtypes, OX1R, and OX2R. Beside these central effects, orexins also play a role in various peripheral organs such as the intestine, pancreas, adrenal glands, kidney, adipose tissue and reproductive tract.In the past few years, an unexpected anti-tumoral role of orexins mediated by a new signaling pathway involving the presence of two immunoreceptor tyrosine-based inhibitory motifs (ITIM) in both orexin receptors subtypes, the recruitment of the phosphotyrosine phosphatase SHP2 and the induction of mitochondrial apoptosis has been elucidated. In the present review, we will discuss the anti-tumoral effect of orexin/OXR system in colon, pancreas, prostate and other cancers, and its interest as a possible therapeutic target.
Keywords: GPCR; cancer; gastroenterology; inflammation; neuropeptides; orexins; signaling pathway.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

