Acute Myeloid Leukemia Cells Express ICOS Ligand to Promote the Expansion of Regulatory T Cells
- PMID: 30319662
- PMCID: PMC6168677
- DOI: 10.3389/fimmu.2018.02227
Acute Myeloid Leukemia Cells Express ICOS Ligand to Promote the Expansion of Regulatory T Cells
Abstract
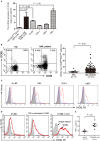
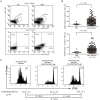
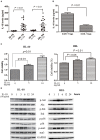
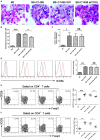
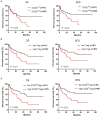
CD4+CD25+Foxp3+ regulatory T cells (Tregs) accumulate in bone marrow microenvironment in acute myeloid leukemia (AML). However, little is known about how the tumor environment including tumor cells themselves affects this process. Here we demonstrated that AML cells expressed inducible T-cell costimulator ligand (ICOSL) that can provide costimulation through ICOS for the conversion and expansion of Tregs sustaining high Foxp3 and CD25 expression as well as a suppressive function. TNF-a stimulation up-regulated the expression of ICOSL. Furthermore, both the conversion and expansion of CD4+CD25+Foxp3+ T cells and CD4+ICOS+Foxp3+ T cells were induced by co-culture with AML cells overexpressed ICOSL. CD4+CD25+ICOS+ T cells possessed stronger ability to secrete IL-10 than CD4+CD25+ICOS- T cells. The mechanism by which IL-10 promoted the proliferation of AML cells was dependent on the activation of the Akt, Erk1/2, p38, and Stat3 signaling pathways. Blockade of ICOS signaling using anti-ICOSL antibody impaired the generation of Tregs and retarded the progression of an AML mice model injected with C1498 cells. The expression of ICOSL of patient AML cells and ICOS+ Tregs were found to be predictors for overall survival and disease-free survival in patients with AML, with ICOS+ Treg cell subset being a stronger predictor than total Tregs. These results suggest that ICOSL expression by AML cells may directly drive Treg expansion as a mechanism of immune evasion and ICOS+ Treg cell frequency is a better prognostic predictor in patients with AML.
Keywords: acute myeloid leukemia; inducible T-cell costimulator; inducible T-cell costimulator ligand; interleukin-10; regulatory T cells.
Figures

References
-
- Maynadie M, Girodon F, Manivet-Janoray I, Mounier M, Mugneret F, Bailly F, et al. . Twenty-five years of epidemiological recording on myeloid malignancies: data from the specialized registry of hematologic malignancies of Cote d'Or (Burgundy, France). Haematologica (2011) 96:55–61. 10.3324/haematol.2010.026252 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

