Ibrutinib Resistance Is Reduced by an Inhibitor of Fatty Acid Oxidation in Primary CLL Lymphocytes
- PMID: 30319974
- PMCID: PMC6168640
- DOI: 10.3389/fonc.2018.00411
Ibrutinib Resistance Is Reduced by an Inhibitor of Fatty Acid Oxidation in Primary CLL Lymphocytes
Abstract
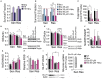
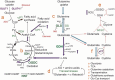
Chronic Lymphocytic Leukemia (CLL) is an incurable disease, characterized by the accumulation of malignant B-lymphocytes in the blood stream (quiescent state) and homing tissues (where they can proliferate). In CLL, the targeting of B-cell receptor signaling through a Burton's tyrosine kinase inhibitor (ibrutinib) has rendered outstanding clinical results. However, complete remission is not guaranteed due to drug resistance or relapse, revealing the need for novel approaches for CLL treatment. The characterization of metabolic rewiring in proliferative cancer cells is already being applied for diagnostic and therapeutic purposes, but our knowledge of quiescent cell metabolism-relevant for CLL cells-is still fragmentary. Recently, we reported that glutamine metabolism in primary CLL cells bearing the del11q deletion is different from their del11q negative counterparts, making del11q cells especially sensitive to glutaminase and glycolysis inhibitors. In this work, we used our primary CLL lymphocyte bank and compounds interfering with central carbon metabolism to define metabolic traits associated with ibrutinib resistance. We observe a differential basal metabolite uptake linked to ibrutinib resistance, favoring glutamine uptake and catabolism. Upon ibrutinib treatment, the redox balance in ibrutinib resistant cells is shifted toward NADPH accumulation, without an increase in glutamine uptake, suggesting alternative metabolic rewiring such as the activation of fatty acid oxidation. In accordance to this idea, the curtailing of fatty acid oxidation by CPT1 inhibition (etomoxir) re-sensitized resistant cells to ibrutinib. Our results suggest that fatty acid oxidation could be explored as a target to overcome ibrutinib resistance.
Keywords: CLL; del17p; drug resistance; fatty acid oxidation; ibrutinib; metabolism.
Figures



References
-
- Oscier DG, Gardiner AC, Mould SJ, Glide S, Davis ZA, Ibbotson RE, et al. . Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood (2002) 100:1177–84. - PubMed
-
- Burger J. B-cell receptor signaling in chronic lymphocytic leukemia and other B-cell malignancies. Clin Adv Hematol Oncol. (2016) 14:55–65. - PubMed
LinkOut - more resources
Full Text Sources

