Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury
- PMID: 30320222
- PMCID: PMC6178585
- DOI: 10.1128/mSystems.00137-18
Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury
Abstract
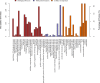
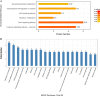
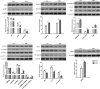
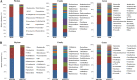
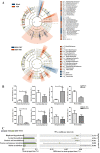
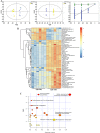
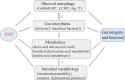
Fecal microbiota transplantation (FMT) is one of the most effective ways to regulate the gut microbiota. Here, we investigated the effect of exogenous fecal microbiota on gut function from the perspective of analysis of the mucosal proteomes in a piglet model. A total of 289 differentially expressed proteins were annotated with 4,068 gene ontology (GO) function entries in the intestinal mucosa, and the levels of autophagy-related proteins in the forkhead box O (FoxO) signaling pathway were increased whereas the levels of proteins related to inflammation response were decreased in the recipient. Then, to assess the alleviation of epithelial injury in the Escherichia coli K88-infected piglets following FMT, intestinal microbiome-metabolome responses were determined. 16S rRNA gene sequencing showed that the abundances of beneficial bacteria, such as Lactobacillus and Succinivibrio, were increased whereas those of Enterobacteriaceae and Proteobacteria bacteria were decreased in the infected piglets following FMT. Metabolomic analysis revealed that levels of 58 metabolites, such as lactic acid and succinic acid, were enhanced in the intestinal lumen and that seven metabolic pathways, such as branched-chain amino acid metabolism pathways, were upregulated in the infected piglets following FMT. In concordance with the metabolome data, results of metagenomics prediction analysis also demonstrated that FMT modulated the metabolic functions of gut microbiota associated with linoleic acid metabolism. In addition, intestinal morphology was improved, a result that coincided with the decrease of intestinal permeability and the enhancement of mucins and mucosal expression of tight junction proteins in the recipient. Taken together, the results showed that FMT triggered intestinal mucosal protective autophagy and alleviated gut barrier injury through alteration of the gut microbial structure. IMPORTANCE The gut microbiota plays a crucial role in human and animal health, and its disorder causes multiple diseases. Over the past decade, FMT has gained increasing attention due to the success in treating Clostridium difficile infection (CDI) and inflammatory bowel disease (IBD). Although FMT appears to be effective, how FMT functions in the recipient remains unknown. Whether FMT exerts this beneficial effect through a series of changes in the host organism caused by alteration of gut microbial structure is also not known. In the present study, newborn piglets and E. coli K88-infected piglets were selected as models to explore the interplay between host and gut microbiota following FMT. Our results showed that FMT triggered intestinal mucosal autophagy and alleviated gut barrier injury caused by E. coli K88. This report provides a theoretical basis for the use of FMT as a viable therapeutic method for gut microbial regulation.
Keywords: autophagy; fecal microbiota transplantation; gut barrier; gut microbiota; piglets.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous
