Voltage-gated calcium channels: their discovery, function and importance as drug targets
- PMID: 30320224
- PMCID: PMC6179141
- DOI: 10.1177/2398212818794805
Voltage-gated calcium channels: their discovery, function and importance as drug targets
Abstract
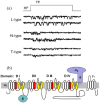
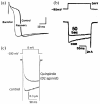
This review will first describe the importance of Ca2+ entry for function of excitable cells, and the subsequent discovery of voltage-activated calcium conductances in these cells. This finding was rapidly followed by the identification of multiple subtypes of calcium conductance in different tissues. These were initially termed low- and high-voltage activated currents, but were then further subdivided into L-, N-, PQ-, R and T-type calcium currents on the basis of differing pharmacology, voltage-dependent and kinetic properties, and single channel conductance. Purification of skeletal muscle calcium channels allowed the molecular identification of the pore-forming and auxiliary α2δ, β and ϒ subunits present in these calcium channel complexes. These advances then led to the cloning of the different subunits, which permitted molecular characterisation, to match the cloned channels with physiological function. Studies with knockout and other mutant mice then allowed further investigation of physiological and pathophysiological roles of calcium channels. In terms of pharmacology, cardiovascular L-type channels are targets for the widely used antihypertensive 1,4-dihydropyridines and other calcium channel blockers, N-type channels are a drug target in pain, and α2δ-1 is the therapeutic target of the gabapentinoid drugs, used in neuropathic pain. Recent structural advances have allowed a deeper understanding of Ca2+ permeation through the channel pore and the structure of both the pore-forming and auxiliary subunits. Voltage-gated calcium channels are subject to multiple pathways of modulation by G-protein and second messenger regulation. Furthermore their trafficking pathways, subcellular localisation and functional specificity are the subjects of active investigation.
Keywords: calcium; channel; heart; neuron; second messenger; voltage.
Conflict of interest statement
Conflict of Interest The author declares there is no conflict of interest.
Figures

References
-
- Baig SM, Koschak A, Lieb A, et al. (2011) Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nature Neuroscience 14: 77–84. - PubMed
-
- Barrett CF, Tsien RW. (2004) Brief history of calcium channel discovery. In: Zamponi GW. (ed.) Voltage-Gated Calcium Channels. Dordrecht: Kluwer Academic/Plenum Publishers, pp. 1–21.
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. (1998) Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genetics 19: 264–267. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

