Reporter Selection for Nanotags in Multiplexed Surface Enhanced Raman Spectroscopy Assays
- PMID: 30320250
- PMCID: PMC6173495
- DOI: 10.1021/acsomega.8b01499
Reporter Selection for Nanotags in Multiplexed Surface Enhanced Raman Spectroscopy Assays
Abstract
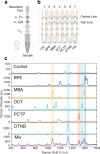
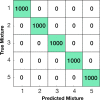
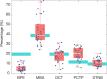
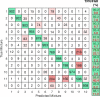
We report a quantitative evaluation of the choice of reporters for multiplexed surface-enhanced Raman spectroscopy (SERS). An initial library consisted of 15 reporter molecules that included commonly used Raman dyes, thiolated reporters, and other small molecules. We used a correlation matrix to downselect Raman reporters from the library to choose five candidates: 1,2-bis(4-pyridyl)ethylene, 4-mercaptobenzoic acid, 3,5-dichlorobenzenthiol, pentachlorothiophenol, and 5,5'-dithiobis(2-nitrobenzoic acid). We evaluated the ability to distinguish the five SERS reporters in a dipstick immunoassay for the biomarker human IgG. Raman nanotags, or gold nanostars conjugated to the five reporters and anti-human IgG polyclonal antibodies were constructed. A linear discriminant analysis approach was used to evaluate the separation of the nanotag spectra in mixtures of fixed ratios.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
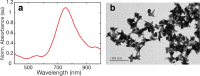
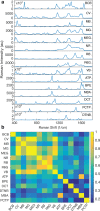
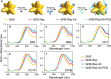
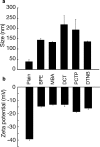


References
-
- Zeman E. J.; Schatz G. C. An Accurate Electromagnetic Theory Study of Surface Enhancement Factors for Silver, Gold, Copper, Lithium, Sodium, Aluminum, Gallium, Indium, Zinc, and Cadmium. J. Phys. Chem. 1987, 91, 634–643. 10.1021/j100287a028. - DOI
-
- Fabris L. Gold-Based Sers Tags for Biomedical Imaging. J. Opt. 2015, 17, 114002.10.1088/2040-8978/17/11/114002. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

