C-3- and C-4-Substituted Bicyclic Coumarin Sulfamates as Potent Steroid Sulfatase Inhibitors
- PMID: 30320251
- PMCID: PMC6173509
- DOI: 10.1021/acsomega.8b01383
C-3- and C-4-Substituted Bicyclic Coumarin Sulfamates as Potent Steroid Sulfatase Inhibitors
Abstract
Synthetic routes to potent bicyclic nonsteroidal sulfamate-based active-site-directed inhibitors of the enzyme steroid sulfatase (STS), an emerging target in the treatment of postmenopausal hormone-dependent diseases, including breast cancer, are described. Sulfamate analogs 9-27 and 28-46 of the core in vivo active two-ring coumarin template, modified at the 4- and 3-positions, respectively, were synthesized to expand structure-activity relationships. α-Alkylacetoacetates were used to synthesize coumarin sulfamate derivatives with 3-position modifications, and the bicyclic ring of other parent coumarins was primarily constructed via the Pechmann synthesis of hydroxyl coumarins. Compounds were examined for STS inhibition in intact MCF-7 breast cancer cells and in placental microsomes. Low nanomolar potency STS inhibitors were achieved, and some were found to inhibit the enzyme in MCF-7 cells ca. 100-500 more potently than the parent 4-methylcoumarin-7-O-sulfamate 3, with the best compounds close in potency to the tricyclic clinical drug Irosustat. 3-Hexyl-4-methylcoumarin-7-O-sulfamate 29 and 3-benzyl-4-methylcoumarin-7-O-sulfamate 41 were particularly effective inhibitors with IC50 values of 0.68 and 1 nM in intact MCF-7 cells and 8 and 32 nM for placental microsomal STS, respectively. They were docked into the STS active site for comparison with estrone 3-O-sulfamate and Irosustat, showing their sulfamate group close to the catalytic hydrated formylglycine residue and their pendant group lying between the hydrophobic sidechains of L103, F178, and F488. Such highly potent STS inhibitors expand the structure-activity relationship for these coumarin sulfamate-based agents that possess therapeutic potential and may be worthy of further development.
Conflict of interest statement
The authors declare the following competing financial interest(s): B.V.L.P. was a Director and stockholder of Sterix Ltd and received research funding that supported some aspects of this work. A.P. was a stockholder of Sterix Ltd.
Figures


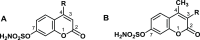






Similar articles
-
Steroidal and nonsteroidal sulfamates as potent inhibitors of steroid sulfatase.J Med Chem. 1998 Mar 26;41(7):1068-83. doi: 10.1021/jm970527v. J Med Chem. 1998. PMID: 9544207
-
Synthesis and evaluation of analogues of estrone-3-O-sulfamate as potent steroid sulfatase inhibitors.Bioorg Med Chem. 2012 Apr 15;20(8):2506-19. doi: 10.1016/j.bmc.2012.03.007. Epub 2012 Mar 8. Bioorg Med Chem. 2012. PMID: 22455789
-
Synthesis and biological evaluation of fluorinated N-benzoyl and N-phenylacetoyl derivatives of 3-(4-aminophenyl)-coumarin-7-O-sulfamate as steroid sulfatase inhibitors.Eur J Med Chem. 2017 Mar 10;128:79-87. doi: 10.1016/j.ejmech.2017.01.028. Epub 2017 Jan 22. Eur J Med Chem. 2017. PMID: 28152429
-
Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential.J Steroid Biochem Mol Biol. 2015 Sep;153:160-9. doi: 10.1016/j.jsbmb.2015.03.012. Epub 2015 Apr 2. J Steroid Biochem Mol Biol. 2015. PMID: 25843211 Review.
-
Sulfamates and their therapeutic potential.Med Res Rev. 2005 Mar;25(2):186-228. doi: 10.1002/med.20021. Med Res Rev. 2005. PMID: 15478125 Review.
Cited by
-
Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest.J Org Chem. 2021 May 7;86(9):6351-6360. doi: 10.1021/acs.joc.1c00172. Epub 2021 Apr 19. J Org Chem. 2021. PMID: 33872504 Free PMC article.
-
Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure-Activity Relationships.Biomedicines. 2024 May 27;12(6):1192. doi: 10.3390/biomedicines12061192. Biomedicines. 2024. PMID: 38927399 Free PMC article. Review.
-
Development of Sulfamoylated 4-(1-Phenyl-1H-1,2,3-triazol-4-yl)phenol Derivatives as Potent Steroid Sulfatase Inhibitors for Efficient Treatment of Breast Cancer.J Med Chem. 2022 Mar 24;65(6):5044-5056. doi: 10.1021/acs.jmedchem.1c02220. Epub 2022 Mar 2. J Med Chem. 2022. PMID: 35235747 Free PMC article.
-
Synthesis of 7-Aminocoumarins from 7-Hydroxycoumarins via Amide Smiles Rearrangement.ACS Omega. 2022 Sep 21;7(39):35269-35279. doi: 10.1021/acsomega.2c04653. eCollection 2022 Oct 4. ACS Omega. 2022. PMID: 36211046 Free PMC article.
-
Recent progress in the development of steroid sulphatase inhibitors - examples of the novel and most promising compounds from the last decade.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1163-1184. doi: 10.1080/14756366.2020.1758692. J Enzyme Inhib Med Chem. 2020. PMID: 32363947 Free PMC article. Review.
References
-
- Dixon J. M. Endocrine Resistance in Breast Cancer. New Journal of Science 2014, 39061810.1155/2014/390618. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

