Asymmetric Entry into 10 b-aza-Analogues of Amaryllidaceae Alkaloids Reveals a Pronounced Electronic Effect on Antiviral Activity
- PMID: 30320263
- PMCID: PMC6173499
- DOI: 10.1021/acsomega.8b01987
Asymmetric Entry into 10 b-aza-Analogues of Amaryllidaceae Alkaloids Reveals a Pronounced Electronic Effect on Antiviral Activity
Abstract
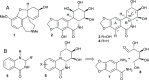
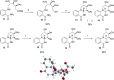
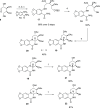
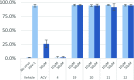
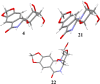
Development of a chiral pool-based synthesis of 10b-aza-analogues of biologically active Amaryllidaceae alkaloids is described, involving a concise reductive amination and condensation sequence, leading to ring-B/C-modified, fully functionalized ring-C derivatives. Differentiated anticancer and antiviral activities of these analogues are presented. Despite complete conformational and functional group overlap, the 10b-aza-analogues have diminished anticancer activity and no antiviral activity. These unprecedented electronic effects suggest a possible role for π-type secondary orbital interactions with the biological target.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
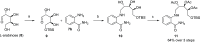
References
-
- Bastida J.; Berkov S.; Pigni N. B.; de Andrade J. P.; Martínez V.; Codina C.; Viladomat F.. Chemical and Biological Aspects of Amaryllidaceae Alkaloids. In Recent Advances in Pharmaceutical Sciences; Muñoz-Torrero D., Ed.; 2011; pp 65–100.
-
- Evidente A.; Kireev A.; Jenkins A.; Romero A.; Steelant W.; Van Slambrouck S.; Kornienko A. Biological Evaluation of Structurally Diverse Amaryllidaceae Alkaloids and Their Synthetic Derivatives: Discovery of Novel Leads for Anticancer Drug Design. Planta Med. 2009, 75, 501–507. 10.1055/s-0029-1185340. - DOI - PMC - PubMed
-
- Martin S. F.The Amaryllidacea Alkaloids. In The Alkaloids; Brossi A., Ed.; Cambridge, Mass., 1987; Vol. 30, pp 251–376.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

