Variability and stability of large-scale cortical oscillation patterns
- PMID: 30320295
- PMCID: PMC6175693
- DOI: 10.1162/netn_a_00046
Variability and stability of large-scale cortical oscillation patterns
Abstract
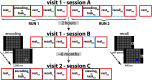
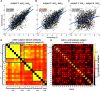
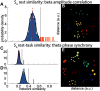
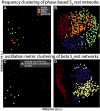
Individual differences in brain organization exist at many spatiotemporal scales and underlie the diversity of human thought and behavior. Oscillatory neural activity is crucial for these processes, but how such rhythms are expressed across the cortex within and across individuals is poorly understood. We conducted a systematic characterization of brain-wide activity across frequency bands and oscillatory features during rest and task execution. We found that oscillatory profiles exhibit sizable group-level similarities, indicating the presence of common templates of oscillatory organization. Nonetheless, well-defined subject-specific network profiles were discernible beyond the structure shared across individuals. These individualized patterns were sufficiently stable to recognize individuals several months later. Moreover, network structure of rhythmic activity varied considerably across distinct oscillatory frequencies and features, indicating the existence of several parallel information processing streams embedded in distributed electrophysiological activity. These findings suggest that network similarity analyses may be useful for understanding the role of large-scale brain oscillations in physiology and behavior.
Keywords: EEG; Functional connectivity; Individual differences; Networks; Oscillations.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures

Similar articles
-
Oscillation-Based Connectivity Architecture Is Dominated by an Intrinsic Spatial Organization, Not Cognitive State or Frequency.J Neurosci. 2021 Jan 6;41(1):179-192. doi: 10.1523/JNEUROSCI.2155-20.2020. Epub 2020 Nov 17. J Neurosci. 2021. PMID: 33203739 Free PMC article.
-
Brain functional connectivity and the pathophysiology of schizophrenia.Psychiatriki. 2014 Apr-Jun;25(2):91-4. Psychiatriki. 2014. PMID: 25035177 English, Greek, Modern.
-
Task- and stimulus-related cortical networks in language production: Exploring similarity of MEG- and fMRI-derived functional connectivity.Neuroimage. 2015 Oct 15;120:75-87. doi: 10.1016/j.neuroimage.2015.07.017. Epub 2015 Jul 11. Neuroimage. 2015. PMID: 26169324
-
The slowed brain: cortical oscillatory activity in hepatic encephalopathy.Arch Biochem Biophys. 2013 Aug 15;536(2):197-203. doi: 10.1016/j.abb.2013.04.004. Epub 2013 Apr 16. Arch Biochem Biophys. 2013. PMID: 23603113 Review.
-
Functional connectivity and oscillatory neuronal activity in the resting human brain.Neuroscience. 2013 Jun 14;240:297-309. doi: 10.1016/j.neuroscience.2013.02.032. Epub 2013 Mar 15. Neuroscience. 2013. PMID: 23500172 Review.
Cited by
-
[Review on identity feature extraction methods based on electroencephalogram signals].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Dec 25;38(6):1203-1210. doi: 10.7507/1001-5515.202102057. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 34970904 Free PMC article. Review. Chinese.
-
Aperiodic Neural Activity is a Better Predictor of Schizophrenia than Neural Oscillations.Clin EEG Neurosci. 2023 Jul;54(4):434-445. doi: 10.1177/15500594231165589. Epub 2023 Jun 7. Clin EEG Neurosci. 2023. PMID: 37287239 Free PMC article.
-
Inter- and Intra-individual Variability in Brain Oscillations During Sports Motor Imagery.Front Hum Neurosci. 2020 Oct 30;14:576241. doi: 10.3389/fnhum.2020.576241. eCollection 2020. Front Hum Neurosci. 2020. PMID: 33192406 Free PMC article.
-
Accounting for Heterogeneity: Mixed-Effects Models in Resting-State EEG Data in a Sample of Tinnitus Sufferers.Brain Topogr. 2020 Jul;33(4):413-424. doi: 10.1007/s10548-020-00772-7. Epub 2020 Apr 23. Brain Topogr. 2020. PMID: 32328859 Free PMC article.
-
Alpha connectivity and inhibitory control in adults with autism spectrum disorder.Mol Autism. 2020 Dec 7;11(1):95. doi: 10.1186/s13229-020-00400-y. Mol Autism. 2020. PMID: 33287904 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
