Cytokine-induced alterations of BAMBI mediate the reciprocal regulation of human Th17/Treg cells in response to cigarette smoke extract
- PMID: 30320351
- PMCID: PMC6202106
- DOI: 10.3892/ijmm.2018.3919
Cytokine-induced alterations of BAMBI mediate the reciprocal regulation of human Th17/Treg cells in response to cigarette smoke extract
Abstract
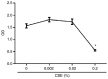
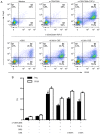
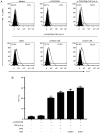
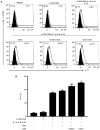
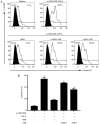
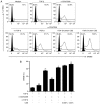
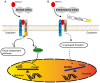
In CD4+ T helper (Th) cells, transforming growth factor β (TGF‑β) is indispensable for the induction of both regulatory T (Treg) and interleukin‑17‑producing effector T helper (Th17) cells. Although BMP and activin membrane‑bound inhibitor (BAMBI) is part of a rheostat‑like mechanism for the regulation of TGF‑β signalling and autoimmune arthritis in mouse models, the underlying activity of BAMBI on the human Th17/Treg cell axis, particularly during exposure to cigarette smoke, remains to be elucidated. The present study aimed to further characterize BAMBI expression in human CD4+ cells, as well as immune imbalance during activation and cigarette smoke exposure. Results from the present study indicated that exposure to cigarette smoke extract partially suppressed Treg differentiation and promoted Th17 cell generation under stimulation by anti‑CD3/28 antibodies and TGF‑β1. Additionally, exposure to cigarette smoke induced an inhibition of phosphorylated‑Smad2/Smad3, which may have arisen from a concomitant enhancement of BAMBI expression. In conclusion, human BAMBI may function as a molecular switch to control TGF‑β signalling strength and the Th17/Treg cell balance, which may be used not only as a biomarker but also as a target of new treatment strategies for maintaining immune tolerance and for the treatment of smoking‑induced immune disorders.
Figures
Similar articles
-
Bone Morphogenetic Protein and Activin Membrane-Bound Inhibitor, a Transforming Growth Factor β Rheostat That Controls Murine Treg Cell/Th17 Cell Differentiation and the Development of Autoimmune Arthritis by Reducing Interleukin-2 Signaling.Arthritis Rheumatol. 2016 Jun;68(6):1551-62. doi: 10.1002/art.39557. Arthritis Rheumatol. 2016. PMID: 26714180
-
BAMBI regulates macrophages inducing the differentiation of Treg through the TGF-β pathway in chronic obstructive pulmonary disease.Respir Res. 2019 Feb 6;20(1):26. doi: 10.1186/s12931-019-0988-z. Respir Res. 2019. PMID: 30728014 Free PMC article.
-
TGF-β/BAMBI pathway dysfunction contributes to peripheral Th17/Treg imbalance in chronic obstructive pulmonary disease.Sci Rep. 2016 Aug 23;6:31911. doi: 10.1038/srep31911. Sci Rep. 2016. PMID: 27549738 Free PMC article.
-
Intricacies of TGF-β signaling in Treg and Th17 cell biology.Cell Mol Immunol. 2023 Sep;20(9):1002-1022. doi: 10.1038/s41423-023-01036-7. Epub 2023 May 23. Cell Mol Immunol. 2023. PMID: 37217798 Free PMC article. Review.
-
Molecular Mechanisms of the Action of Vitamin A in Th17/Treg Axis in Multiple Sclerosis.J Mol Neurosci. 2015 Dec;57(4):605-13. doi: 10.1007/s12031-015-0643-1. Epub 2015 Aug 30. J Mol Neurosci. 2015. PMID: 26319266 Review.
Cited by
-
Abhd2, a Candidate Gene Regulating Airway Remodeling in COPD via TGF-β.Int J Chron Obstruct Pulmon Dis. 2024 Jan 5;19:33-50. doi: 10.2147/COPD.S440200. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38197032 Free PMC article.
-
Smoking and osteoimmunology: Understanding the interplay between bone metabolism and immune homeostasis.J Orthop Translat. 2024 May 10;46:33-45. doi: 10.1016/j.jot.2024.04.003. eCollection 2024 May. J Orthop Translat. 2024. PMID: 38765605 Free PMC article. Review.
References
-
- Postigo J, Iglesias M, Alvarez P, Jesús Augustin J, Buelta L, Merino J, Merino R. Bone morphogenetic protein and activin membrane-bound inhibitor, a transforming growth factor β rheostat that controls murine treg Cell/Th17 cell differentiation and the development of autoimmune arthritis by reducing interleukin-2 signaling. Arthritis Rheumatol. 2016;68:1551–1562. doi: 10.1002/art.39557. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

