Interleukin‑10 promotes proliferation and migration, and inhibits tendon differentiation via the JAK/Stat3 pathway in tendon‑derived stem cells in vitro
- PMID: 30320384
- PMCID: PMC6236255
- DOI: 10.3892/mmr.2018.9547
Interleukin‑10 promotes proliferation and migration, and inhibits tendon differentiation via the JAK/Stat3 pathway in tendon‑derived stem cells in vitro
Abstract
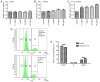
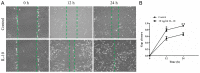
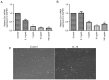
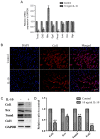
Tendon repair follows a slow course of early inflammatory, proliferative and remodeling phases, which commonly results in the failure and loss of normal biomechanical properties. Previous studies have demonstrated that tendon‑derived stem cells (TDSCs) are vital healing cells and that mRNA expression of anti‑inflammatory cytokine interleukin (IL)‑10 is significantly upregulated at the late inflammatory phase. To explore how IL‑10 may impact tendon healing, the present study investigated the in vitro effects of IL‑10 on TDSCs isolated from rat Achilles tendons. Cellular activities of TDSCs and the expression levels of tendon cell markers were measured treatment with IL‑10 and subsequent performance of wound healing assays, reverse transcription‑quantitative polymerase chain reaction and western blot analyses. The results demonstrated that IL‑10 treatment markedly increased the proliferative capacity of TDSCs. In addition, IL‑10 significantly enhanced cell migration when compared with the control cells. Furthermore, IL‑10 treatment significantly activated the JAK/Stat3 signaling pathway and inhibited the protein expression of tendon cell markers, including scleraxis and tenomodulin. Notably, IL‑10 treatment also reduced the gene expression levels of type 1 collagen, type 3 collagen, lumican and fibromodulin in TDSCs. These findings indicated that IL‑10 enhanced cell proliferation and migration, and inhibited tenogenic differentiation in TDSCs in vitro. Reducing the negative effects whilst enhancing the positive effects of IL‑10 may be a potential therapeutic target in tendon repair.
Keywords: interleukin‑10; tendon; tendon‑derived stem cells; injury.
Figures
Similar articles
-
Interleukin-6 Promotes Proliferation but Inhibits Tenogenic Differentiation via the Janus Kinase/Signal Transducers and Activators of Transcription 3 (JAK/STAT3) Pathway in Tendon-Derived Stem Cells.Med Sci Monit. 2018 Mar 16;24:1567-1573. doi: 10.12659/msm.908802. Med Sci Monit. 2018. PMID: 29547593 Free PMC article.
-
Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering.Stem Cells Dev. 2012 Mar 20;21(5):790-800. doi: 10.1089/scd.2011.0160. Epub 2011 Aug 8. Stem Cells Dev. 2012. PMID: 21627568 Free PMC article.
-
The 532 nm Laser Treatment Promotes the Proliferation of Tendon-Derived Stem Cells and Upregulates Nr4a1 to Stimulate Tenogenic Differentiation.Photobiomodul Photomed Laser Surg. 2022 Aug;40(8):543-553. doi: 10.1089/photob.2022.0003. Epub 2022 Jul 28. Photobiomodul Photomed Laser Surg. 2022. PMID: 35904935
-
Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications.Stem Cell Rev Rep. 2011 Nov;7(4):883-97. doi: 10.1007/s12015-011-9276-0. Stem Cell Rev Rep. 2011. PMID: 21611803 Review.
-
Tendon-derived stem cells as a new cell source for tendon tissue engineering.Front Biosci (Landmark Ed). 2013 Jan 1;18(2):756-64. doi: 10.2741/4138. Front Biosci (Landmark Ed). 2013. PMID: 23276960 Review.
Cited by
-
Challenges and perspectives of tendon-derived cell therapy for tendinopathy: from bench to bedside.Stem Cell Res Ther. 2022 Sep 2;13(1):444. doi: 10.1186/s13287-022-03113-6. Stem Cell Res Ther. 2022. PMID: 36056395 Free PMC article. Review.
-
Erroneous Differentiation of Tendon Stem/Progenitor Cells in the Pathogenesis of Tendinopathy: Current Evidence and Future Perspectives.Stem Cell Rev Rep. 2025 Feb;21(2):423-453. doi: 10.1007/s12015-024-10826-z. Epub 2024 Nov 23. Stem Cell Rev Rep. 2025. PMID: 39579294 Review.
-
Characterization of Tendon-Derived Stem Cells and Rescue Tendon Injury.Stem Cell Rev Rep. 2021 Oct;17(5):1534-1551. doi: 10.1007/s12015-021-10143-9. Epub 2021 Mar 2. Stem Cell Rev Rep. 2021. PMID: 33651334 Review.
-
Role of tendon-derived stem cells in tendon and ligament repair: focus on tissue engineer.Front Bioeng Biotechnol. 2024 Aug 8;12:1357696. doi: 10.3389/fbioe.2024.1357696. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39175617 Free PMC article. Review.
-
The role of fibromodulin in inflammatory responses and diseases associated with inflammation.Front Immunol. 2023 Jul 7;14:1191787. doi: 10.3389/fimmu.2023.1191787. eCollection 2023. Front Immunol. 2023. PMID: 37483637 Free PMC article. Review.
References
-
- Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

