Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells
- PMID: 30320388
- PMCID: PMC6278372
- DOI: 10.3892/or.2018.6780
Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells
Erratum in
-
[Corrigendum] Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells.Oncol Rep. 2020 Sep;44(3):1293. doi: 10.3892/or.2020.7653. Epub 2020 Jun 19. Oncol Rep. 2020. PMID: 32582993 Free PMC article.
Abstract
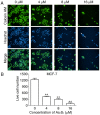
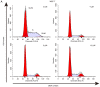
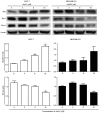
In the present study, the antitumor effects of arsenic disulfide (As2S2) on the proliferative, survival and migratory ability of human breast cancer MCF‑7 and MDA‑MB‑231 cells were investigated, and its potential underlying molecular mechanisms with an emphasis on cell cycle arrest, apoptosis induction, autophagy induction and reactive oxygen species (ROS) generation were determined. The results indicated that As2S2 significantly inhibited the viability, survival and migration of breast cancer cells in a dose‑dependent manner. In addition, it was identified that As2S2 induced cell cycle arrest primarily at G2/M phase in the two breast cancer cell lines by regulating the expression of associated proteins, including cyclin B1 and cell division cycle protein 2. In addition to cell cycle arrest, As2S2 also triggered the induction of apoptosis in cells by activating the expression of pro‑apoptotic proteins, including caspase‑7 and ‑8, as well as increasing the B‑cell lymphoma 2 (Bcl‑2)‑associated X protein/Bcl‑2 ratio, while decreasing the protein expression of anti‑apoptotic B‑cell lymphoma extra‑large. In addition, As2S2 stimulated the accumulation of microtubule‑associated protein 1A/1B‑light chain 3 (LC3)‑II and increased the LC3‑II/LC3‑I ratio, indicating the occurrence of autophagy. As2S2 treatment also inhibited the protein expression of matrix metalloproteinase‑9 (MMP‑9), but increased the intracellular accumulation of ROS in the two breast cancer cell lines, which may assist in alleviating metastasis and attenuating the progression of breast cancer. Taken together, the results of the present study suggest that As2S2 inhibits the progression of human breast cancer cells through the regulation of cell cycle arrest, intrinsic and extrinsic apoptosis, autophagy, MMP‑9 signaling and ROS generation.
Figures
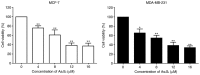













References
-
- Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. - DOI - PMC - PubMed
-
- Zhang QY, Mao JH, Liu P, Huang QH, Lu J, Xie YY, Weng L, Zhang Y, Chen Q, Chen SJ, et al. A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA. 2009;106:3378–3383. doi: 10.1073/pnas.0813142106. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

