Hydrogen molecules (H2) improve perfusion recovery via antioxidant effects in experimental peripheral arterial disease
- PMID: 30320393
- PMCID: PMC6236306
- DOI: 10.3892/mmr.2018.9546
Hydrogen molecules (H2) improve perfusion recovery via antioxidant effects in experimental peripheral arterial disease
Abstract
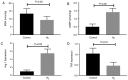
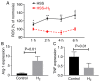
Reactive oxygen species (ROS) impair neovascularization and perfusion recovery following limb ischemia in patients with peripheral arterial disease (PAD). Hydrogen molecules (H2) comprise an antioxidant gas that has been reported to neutralize cytotoxic ROS. The present study investigated whether H2 may serve as a novel therapeutic strategy for PAD. H2‑saturated water or dehydrogenized water was supplied to mice with experimental PAD. Laser Doppler perfusion imaging demonstrated that H2‑saturated water improved perfusion recovery, decreased the rate of necrosis, increased the capillary density in the gastrocnemius muscle and increased the artery density in the abductor muscle in the ischemic limbs, at 14 and 21 days post‑hindlimb ischemia. Ischemic muscle tissue was harvested 7 days after experimental PAD for biochemical testing and H2 was observed to reduce the levels of malondialdehyde and increase the levels of cyclic guanine monophosphate (cGMP). In cultured endothelial cells, H2‑saturated culture medium resulted in reduced ROS levels, increased tube formation and increased cGMP levels. In macrophages, H2 decreased cellular ROS levels and promoted M2 polarization. H2‑saturated water increases angiogenesis and arteriogenesis and subsequently improves perfusion recovery in a mouse PAD model via reduction of ROS levels.
Keywords: hydrogen molecule; reactive oxygen species; peripheralarterial disease; angiogenesis; arteriogenesis.
Figures


Similar articles
-
Sodium Tanshinone IIA sulfonate improves post-ischemic angiogenesis in hyperglycemia.Biochem Biophys Res Commun. 2019 Dec 10;520(3):580-585. doi: 10.1016/j.bbrc.2019.09.106. Epub 2019 Oct 14. Biochem Biophys Res Commun. 2019. PMID: 31623833
-
A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle.Circulation. 2017 Jun 13;135(24):2403-2425. doi: 10.1161/CIRCULATIONAHA.116.025490. Epub 2017 Mar 29. Circulation. 2017. PMID: 28356443 Free PMC article.
-
Interleukin-22 receptor 1 upregulation and activation in hypoxic endothelial cells improves perfusion recovery in experimental peripheral arterial disease.Biochem Biophys Res Commun. 2018 Oct 20;505(1):60-66. doi: 10.1016/j.bbrc.2018.08.163. Epub 2018 Sep 17. Biochem Biophys Res Commun. 2018. PMID: 30236983
-
The roles of interleukins in perfusion recovery after peripheral arterial disease.Biosci Rep. 2018 Feb 13;38(1):BSR20171455. doi: 10.1042/BSR20171455. Print 2018 Feb 28. Biosci Rep. 2018. PMID: 29358309 Free PMC article. Review.
-
Epigenetic regulators of the revascularization response to chronic arterial occlusion.Cardiovasc Res. 2019 Mar 15;115(4):701-712. doi: 10.1093/cvr/cvz001. Cardiovasc Res. 2019. PMID: 30629133 Free PMC article. Review.
Cited by
-
Clinical Potential of Hydrogen Sulfide in Peripheral Arterial Disease.Int J Mol Sci. 2023 Jun 9;24(12):9955. doi: 10.3390/ijms24129955. Int J Mol Sci. 2023. PMID: 37373103 Free PMC article. Review.
-
Exercise Prior to Lower Extremity Peripheral Artery Disease Improves Endurance Capacity and Hindlimb Blood Flow by Inhibiting Muscle Inflammation.Front Cardiovasc Med. 2021 Aug 4;8:706491. doi: 10.3389/fcvm.2021.706491. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34422931 Free PMC article.
-
A review of the current state in neointimal hyperplasia development following endovascular intervention and minor emphasis on new horizons in immunotherapy.Transl Clin Pharmacol. 2023 Dec;31(4):191-201. doi: 10.12793/tcp.2023.31.e18. Epub 2023 Nov 22. Transl Clin Pharmacol. 2023. PMID: 38196998 Free PMC article. Review.
References
-
- Guerchet M, Aboyans V, Mbelesso P, Mouanga AM, Salazar J, Bandzouzi B, Tabo A, Clément JP, Preux PM, Lacroix P. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur J Vasc Endovasc Surg. 2012;44:164–169. doi: 10.1016/j.ejvs.2012.05.019. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

