Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS
- PMID: 30320556
- PMCID: PMC6234026
- DOI: 10.7554/eLife.41973
Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS
Abstract
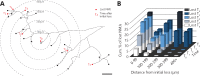
Despite being an early event in ALS, it remains unclear whether the denervation of neuromuscular junctions (NMJ) is simply the first manifestation of a globally degenerating motor neuron. Using in vivo imaging of single axons and their NMJs over a three-month period, we identify that single motor-units are dismantled asynchronously in SOD1G37R mice. We reveal that weeks prior to complete axonal degeneration, the dismantling of axonal branches is accompanied by contemporaneous new axonal sprouting resulting in synapse formation onto nearby NMJs. Denervation events tend to propagate from the first lost NMJ, consistent with a contribution of neuromuscular factors extrinsic to motor neurons, with distal branches being more susceptible. These results show that NMJ denervation in ALS is a complex and dynamic process of continuous denervation and new innervation rather than a manifestation of sudden global motor neuron degeneration.
Keywords: amyotrophic lateral sclerosis; motor unit; mouse; neuromuscular junction; neuroscience; reinnervation; superoxide dismutase (SOD1); synapse loss.
© 2018, Martineau et al.
Conflict of interest statement
ÉM, AD, CV, RR No competing interests declared
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

