Transfer of assembled collagen fibrils to flexible substrates for mechanically tunable contact guidance cues
- PMID: 30320857
- PMCID: PMC6267882
- DOI: 10.1039/c8ib00127h
Transfer of assembled collagen fibrils to flexible substrates for mechanically tunable contact guidance cues
Abstract
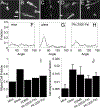
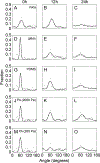
Contact guidance or bidirectional migration along aligned fibers modulates many physiological and pathological processes such as wound healing and cancer invasion. Aligned 2D collagen fibrils epitaxially grown on mica substrates replicate many features of contact guidance seen in aligned 3D collagen fiber networks. However, these 2D collagen self-assembled substrates are difficult to image through, do not have known or tunable mechanical properties and cells degrade and mechanically detach collagen fibrils from the surface, leading to an inability to assess contact guidance over long times. Here, we describe the transfer of aligned collagen fibrils from mica substrates to three different functionalized target substrates: glass, polydimethylsiloxane (PDMS) and polyacrylamide (PA). Aligned collagen fibrils can be efficiently transferred to all three substrates. This transfer resulted in substrates that were to varying degrees resistant to cell-mediated collagen fibril deformation that resulted in detachment of the collagen fibril field, allowing for contact guidance to be observed over longer time periods. On these transferred substrates, cell speed is lowest on softer contact guidance cues for both MDA-MB-231 and MTLn3 cells. Intermediate stiffness resulted in the fastest migration. MTLn3 cell directionality was low on soft contact guidance cues, whereas MDA-MB-231 cell directionality marginally increased. It appears that the stiffness of the contact guidance cue regulates contact guidance differently between cell types. The development of this collagen fibril transfer method allows for the attachment of aligned collagen fibrils on substrates, particularly flexible substrates, that do not normally promote aligned collagen fibril growth, increasing the utility of this collagen self-assembly system for the fundamental examination of mechanical regulation of contact guidance.
Figures

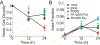

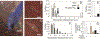


Similar articles
-
Epitaxially grown collagen fibrils reveal diversity in contact guidance behavior among cancer cells.Langmuir. 2015;31(1):307-14. doi: 10.1021/la503254x. Epub 2014 Dec 22. Langmuir. 2015. PMID: 25531276 Free PMC article.
-
Contact guidance diversity in rotationally aligned collagen matrices.Acta Biomater. 2018 Jan 15;66:248-257. doi: 10.1016/j.actbio.2017.11.039. Epub 2017 Nov 28. Acta Biomater. 2018. PMID: 29196116 Free PMC article.
-
Degradation and Remodeling of Epitaxially Grown Collagen Fibrils.Cell Mol Bioeng. 2019 Feb;12(1):69-84. doi: 10.1007/s12195-018-0547-6. Epub 2018 Aug 16. Cell Mol Bioeng. 2019. PMID: 31007771 Free PMC article.
-
Interstitial guidance of cancer invasion.J Pathol. 2012 Jan;226(2):185-99. doi: 10.1002/path.3031. J Pathol. 2012. PMID: 22006671 Review.
-
Quantifying traction stresses in adherent cells.Methods Cell Biol. 2012;110:139-78. doi: 10.1016/B978-0-12-388403-9.00006-0. Methods Cell Biol. 2012. PMID: 22482948 Review.
Cited by
-
Linking cell mechanical memory and cancer metastasis.Nat Rev Cancer. 2024 Mar;24(3):216-228. doi: 10.1038/s41568-023-00656-5. Epub 2024 Jan 18. Nat Rev Cancer. 2024. PMID: 38238471 Free PMC article. Review.
-
Collagen-Based Biomimetic Systems to Study the Biophysical Tumour Microenvironment.Cancers (Basel). 2022 Nov 30;14(23):5939. doi: 10.3390/cancers14235939. Cancers (Basel). 2022. PMID: 36497421 Free PMC article. Review.
-
Substrate Resistance to Traction Forces Controls Fibroblast Polarization.Biophys J. 2020 Dec 15;119(12):2558-2572. doi: 10.1016/j.bpj.2020.10.043. Epub 2020 Nov 18. Biophys J. 2020. PMID: 33217384 Free PMC article.
-
Aligned Collagen Fibers Drive Distinct Traction Force Signatures to Regulate Contact Guidance.ACS Nano. 2025 Aug 26;19(33):30165-30185. doi: 10.1021/acsnano.5c06736. Epub 2025 Aug 14. ACS Nano. 2025. PMID: 40811686 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

