Long-Term Impact of Belimumab on Health-Related Quality of Life and Fatigue in Patients With Systemic Lupus Erythematosus: Six Years of Treatment
- PMID: 30320964
- PMCID: PMC6593666
- DOI: 10.1002/acr.23788
Long-Term Impact of Belimumab on Health-Related Quality of Life and Fatigue in Patients With Systemic Lupus Erythematosus: Six Years of Treatment
Abstract
Objective: To report long-term health-related quality of life (HRQoL) and fatigue outcomes in patients with systemic lupus erythematosus (SLE) receiving belimumab.
Methods: Patients with SLE who completed the Study of Belimumab in Subjects with SLE 76-week trial (BLISS-76) were enrolled in this continuation study (BEL112233 [ClinicalTrials.gov identifier: NCT00724867]). The belimumab groups continued to receive the same dose (1 mg/kg or 10 mg/kg) intravenously. After March 2011, all patients received belimumab 10 mg/kg every 28 days plus standard therapy. The placebo group switched to belimumab 10 mg/kg. HRQoL and fatigue assessments included the Short Form 36 (SF-36) health survey and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue subscale. Post hoc subgroup analyses (BEL206350) assessed clinical characteristics associated with improved HRQoL and fatigue.
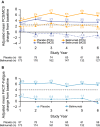
Results: Of the 268 patients enrolled, 140 completed the study. Patients receiving long-term belimumab treatment reported continued improvements in HRQoL and fatigue. At study year 6, the mean ± SD SF-36 physical component summary (PCS) score and the mental component summary (MCS) score increased from 37.0 ± 9.9 at baseline to 41.7 ± 10.0 (mean ± SD change 4.8 ± 9.4) and from 44.3 ± 11.3 to 47.0 ± 11.6 (mean ± SD change 2.7 ± 11.3) for the PCS and MCS, respectively, exceeding the minimum clinically important difference (MCID) for improvement (2.5 units). The mean ± SD FACIT-Fatigue score exceeded the MCID of 4 at study years 1-5; at study year 6, the mean ± SD change was 3.7 ± 11.8. Statistically significant associations were observed between parent trial treatment groups and change from baseline in PCS, MCS, and FACIT-Fatigue scores (P < 0.01).
Conclusion: Long-term control of SLE disease activity with belimumab plus standard therapy translates into meaningful improvements in patient-reported fatigue and HRQoL.
© 2018, GSK. Arthritis Care & Research published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures



References
-
- Gilboe IM, Kvien TK, Husby G. Disease course in systemic lupus erythematosus: changes in health status, disease activity, and organ damage after 2 years. J Rheumatol 2001;28:266–74. - PubMed
-
- Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health‐related quality of life in patients with systemic lupus erythematosus. J Rheumatol 2010;37:568–73. - PubMed
-
- Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology 2012;51:491–8. - PubMed
-
- Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology 2009;48:281–4. - PubMed
-
- Strand V, Chu AD. Generic versus disease‐specific measures of health‐related quality of life in systemic lupus erythematosus. J Rheumatol 2011;38:1821–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

