Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity
- PMID: 30321069
- PMCID: PMC6338644
- DOI: 10.1096/fj.201800529RR
Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity
Abstract
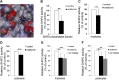
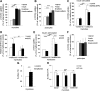
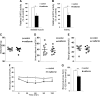
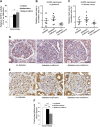
Metformin, the first-line drug to treat type 2 diabetes (T2D), inhibits mitochondrial glycerolphosphate dehydrogenase in the liver to suppress gluconeogenesis. However, the direct target and the underlying mechanisms by which metformin increases glucose uptake in peripheral tissues remain uncharacterized. Lipid phosphatase Src homology 2 domain-containing inositol-5-phosphatase 2 (SHIP2) is upregulated in diabetic rodent models and suppresses insulin signaling by reducing Akt activation, leading to insulin resistance and diminished glucose uptake. Here, we demonstrate that metformin directly binds to and reduces the catalytic activity of the recombinant SHIP2 phosphatase domain in vitro. Metformin inhibits SHIP2 in cultured cells and in skeletal muscle and kidney of db/db mice. In SHIP2-overexpressing myotubes, metformin ameliorates reduced glucose uptake by slowing down glucose transporter 4 endocytosis. SHIP2 overexpression reduces Akt activity and enhances podocyte apoptosis, and both are restored to normal levels by metformin. SHIP2 activity is elevated in glomeruli of patients with T2D receiving nonmetformin medication, but not in patients receiving metformin, compared with people without diabetes. Furthermore, podocyte loss in kidneys of metformin-treated T2D patients is reduced compared with patients receiving nonmetformin medication. Our data unravel a novel molecular mechanism by which metformin enhances glucose uptake and acts renoprotectively by reducing SHIP2 activity.-Polianskyte-Prause, Z., Tolvanen, T. A., Lindfors, S., Dumont, V., Van, M., Wang, H., Dash, S. N., Berg, M., Naams, J.-B., Hautala, L. C., Nisen, H., Mirtti, T., Groop, P.-H., Wähälä, K., Tienari, J., Lehtonen, S. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity.
Keywords: diabetic kidney disease; insulin resistance; lipid phosphatase; podocyte; type 2 diabetes.
Conflict of interest statement
The authors thank M. Saleem (Southmead Hospital, University of Bristol, Bristol United Kingdom) for kindly providing immortalized human podocytes, S. Cushman (National Institutes of Health, Bethesda, MD, USA) for the HA-GLUT4-GFP construct, and C. A. Mitchell (Monash University, Clayton, VIC, Australia) and G. Krystal (Terry Fox Laboratory, Vancouver, BC, Canada) for human SHIP2 (NP_001558) and SHIP1 (NP_005532) cDNA constructs, respectively. Leena Saikko, High Throughput Biomedicine Unit, Biomedicum Flow Cytometry Unit, Biochemical Analysis Core for Experimental Research, and Genome Biology Unit (University of Helsinki) are acknowledged for excellent technical assistance. This work was supported by the European Research Council (Grant 242820; to S.L.), Academy of Finland (Grants 131255, 218021, and 255551 to S.L.; Grant 134379 to P.-H.G.), Jane and Aatos Erkko Foundation (to S.L.), European Foundation for the Study of Diabetes/Boehringer Ingelheim Research Programme (to S.L.), Sigrid Jusélius Foundation (to S.L.), Päivikki and Sakari Sohlberg Foundation (to S.L.), Diabetes Research Foundation (to S.L.), Business Finland (1964/31/2017 to S.L.), Faculty of Medicine, University of Helsinki (to S.L.), Helsinki University Hospital (to J.T.), Doctoral Programme in Biomedicine (to T.A.T. and V.D.), Folkhälsan Research Foundation (to P.-H.G.), and Novo Nordisk Foundation (to P.-H.G.). P.-H.G. has received research grants from Eli Lilly and Roche, and is an advisory board member for AbbVie, AstraZeneca, Boehringer-Ingelheim, Cebix, Eli Lilly, Janssen, Medscape, Musculoskeletal Diseases (MSD), and Novartis. P.-H.G. has received lecture honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Genzyme, MSD, Novartis, Novo Nordisk, and Sanofi. However, none of the above are relevant to the article. The remaining authors declare no conflicts of interest.
Figures

References
-
- Hundal, R. S., Krssak, M., Dufour, S., Laurent, D., Lebon, V., Chandramouli, V., Inzucchi, S. E., Schumann, W. C., Petersen, K. F., Landau, B. R., Shulman, G. I. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 10.2337/diabetes.49.12.2063 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

