Marmoset as a Model to Study Kidney Changes Associated With Aging
- PMID: 30321310
- PMCID: PMC6376089
- DOI: 10.1093/gerona/gly237
Marmoset as a Model to Study Kidney Changes Associated With Aging
Erratum in
-
Corrigendum to: Marmoset as a Model to Study Kidney Changes Associated With Aging.J Gerontol A Biol Sci Med Sci. 2022 Jan 7;77(1):84. doi: 10.1093/gerona/glab238. J Gerontol A Biol Sci Med Sci. 2022. PMID: 34542600 Free PMC article. No abstract available.
Abstract
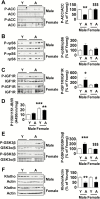
We evaluated whether the marmoset, a nonhuman primate, can serve as a good model to study aging-related changes in the kidney by employing healthy young and aged marmosets of both sexes. Aging was associated with glomerulosclerosis, interstitial fibrosis, and arteriolosclerosis in both sexes; correspondingly, the content of matrix proteins was increased. Functionally, aging resulted in an increase in urinary albumin and protein excretion. There was a robust correlation between markers of fibrosis and functional changes. We explored signaling pathways as potential mechanistic events. Aging in males, but not in females, was associated with reduced renal cortical activity of AMP-activated protein kinase (AMPK) and a trend toward activation of mechanistic target of rapamycin complex 1 (mTORC1); upstream of AMPK and mTORC1, Akt and IGF-1 receptor were activated. In both sexes, aging promoted kidney activation of transforming growth factor β-1 signaling pathway. While the expression of cystathionine β-synthase (CBS), an enzyme involved hydrogen sulfide (H2S) synthesis, was reduced in both aged males and females, decreased H2S generation was seen in only males. Our studies show that the marmoset is a valid model to study kidney aging; some of the signaling pathways involved in renal senescence differ between male and female marmosets.
Keywords: Extracellular matrix; Fibrosis; Glomerulus; Primates; Signaling.
Published by Oxford University Press on behalf of The Gerontological Society of America 2018.
Figures



Similar articles
-
Hydrogen sulfide ameliorates aging-associated changes in the kidney.Geroscience. 2018 Apr;40(2):163-176. doi: 10.1007/s11357-018-0018-y. Epub 2018 May 1. Geroscience. 2018. PMID: 29717417 Free PMC article.
-
Methionine restriction delays senescence and suppresses the senescence-associated secretory phenotype in the kidney through endogenous hydrogen sulfide.Cell Cycle. 2019 Jul;18(14):1573-1587. doi: 10.1080/15384101.2019.1618124. Epub 2019 Jun 5. Cell Cycle. 2019. PMID: 31164038 Free PMC article.
-
Tadalafil Integrates Nitric Oxide-Hydrogen Sulfide Signaling to Inhibit High Glucose-induced Matrix Protein Synthesis in Podocytes.J Biol Chem. 2015 May 8;290(19):12014-26. doi: 10.1074/jbc.M114.615377. Epub 2015 Mar 9. J Biol Chem. 2015. PMID: 25752605 Free PMC article.
-
Protective Effects of Hydrogen Sulfide in the Ageing Kidney.Oxid Med Cell Longev. 2016;2016:7570489. doi: 10.1155/2016/7570489. Epub 2016 Nov 1. Oxid Med Cell Longev. 2016. PMID: 27882191 Free PMC article.
-
Review on testicular development, structure, function, and regulation in common marmoset.Birth Defects Res B Dev Reprod Toxicol. 2005 Oct;74(5):450-69. doi: 10.1002/bdrb.20057. Birth Defects Res B Dev Reprod Toxicol. 2005. PMID: 16193499 Review.
Cited by
-
Factors Affecting Hematologic and Serum Biochemical Parameters in Healthy Common Marmosets (Callithrix jacchus).J Am Assoc Lab Anim Sci. 2022 Mar 1;61(2):113-131. doi: 10.30802/AALAS-JAALAS-21-000061. Epub 2022 Jan 7. J Am Assoc Lab Anim Sci. 2022. PMID: 34996528 Free PMC article.
-
Hydrogen sulfide in ageing, longevity and disease.Biochem J. 2021 Oct 15;478(19):3485-3504. doi: 10.1042/BCJ20210517. Biochem J. 2021. PMID: 34613340 Free PMC article. Review.
-
Proximal tubular epithelial insulin receptor mediates high-fat diet-induced kidney injury.JCI Insight. 2021 Feb 8;6(3):e143619. doi: 10.1172/jci.insight.143619. JCI Insight. 2021. PMID: 33400689 Free PMC article.
-
Clinical Management of Gastrointestinal Disease in the Common Marmoset (Callithrix jacchus).ILAR J. 2020 Dec 31;61(2-3):199-217. doi: 10.1093/ilar/ilab012. ILAR J. 2020. PMID: 33989417 Free PMC article. Review.
-
Pseudomonas Infections in the Common Marmoset (Callithrix jacchus): Gross and Histopathological Findings.J Med Primatol. 2025 Apr;54(2):e70016. doi: 10.1111/jmp.70016. J Med Primatol. 2025. PMID: 40166954
References
-
- Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. - PubMed
-
- Ikeno Y, Hubbard GB, Lee S, et al. . Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. - PubMed
-
- Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–B88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

