GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity
- PMID: 30321352
- PMCID: PMC6734491
- DOI: 10.1093/jmcb/mjy056
GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity
Abstract
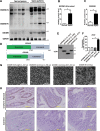
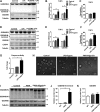
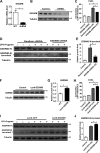
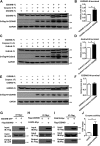
Gasdermin B (GSDMB) has been reported to be associated with immune diseases in humans, but the detailed molecular mechanisms remain unsolved. The N-terminus of GSDMB by itself, unlike other gasdermin family proteins, does not induce cell death. Here, we show that GSDMB is highly expressed in the leukocytes of septic shock patients, which is associated with increased release of the gasdermin D (GSDMD) N-terminus. GSDMB expression and the accumulation of the N-terminal fragment of GSDMD are induced by the activation of the non-canonical pyroptosis pathway in a human monocyte cell line. The downregulation of GSDMB alleviates the cleavage of GSDMD and cell death. Consistently, the overexpression of GSDMB promotes GSDMD cleavage, accompanied by increased LDH release. We further found that GSDMB promotes caspase-4 activity, which is required for the cleavage of GSDMD in non-canonical pyroptosis, by directly binding to the CARD domain of caspase-4. Our study reveals a GSDMB-mediated novel regulatory mechanism for non-canonical pyroptosis and suggests a potential new strategy for the treatment of inflammatory diseases.
Keywords: GSDMB; GSDMD; pyroptosis; sepsis.
© The Author(s) (2018). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. All rights reserved.
Figures