Mucosal bromodomain-containing protein 4 mediates aeroallergen-induced inflammation and remodeling
- PMID: 30321559
- PMCID: PMC6490683
- DOI: 10.1016/j.jaci.2018.09.029
Mucosal bromodomain-containing protein 4 mediates aeroallergen-induced inflammation and remodeling
Abstract
Background: Frequent exacerbations of allergic asthma lead to airway remodeling and a decrease in pulmonary function, producing morbidity. Cat dander is an aeroallergen associated with asthma risk.
Objective: We sought to elucidate the mechanism of cat dander-induced inflammation-remodeling.
Methods: We identified remodeling in mucosal samples from allergic asthma by using quantitative RT-PCR. We developed a model of aeroallergen-induced experimental asthma using repetitive cat dander extract exposure. We measured airway inflammation using immunofluorescence, leukocyte recruitment, and quantitative RT-PCR. Airway remodeling was measured by using histology, collagen content, myofibroblast numbers, and selected reaction monitoring. Inducible nuclear factor κB (NF-κB)-BRD4 interaction was measured by using a proximity ligation assay in situ.
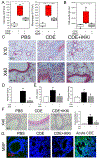
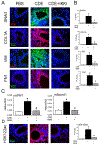
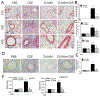
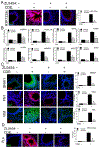
Results: Enhanced mesenchymal signatures are observed in bronchial biopsy specimens from patients with allergic asthma. Cat dander induces innate inflammation through NF-κB signaling, followed by production of a profibrogenic mesenchymal transition in primary human small airway epithelial cells. The IκB kinase-NF-κB signaling pathway is required for mucosal inflammation-coupled airway remodeling and myofibroblast expansion in the mouse model of aeroallergen exposure. Cat dander induces NF-κB/RelA to complex with and activate BRD4, resulting in modifying the chromatin environment of inflammatory and fibrogenic genes through its atypical histone acetyltransferase activity. A novel small-molecule BRD4 inhibitor (ZL0454) disrupts BRD4 binding to the NF-κB-RNA polymerase II complex and inhibits its histone acetyltransferase activity. ZL0454 prevents epithelial mesenchymal transition, myofibroblast expansion, IgE sensitization, and fibrosis in airways of naive mice exposed to cat dander.
Conclusions: NF-κB-inducible BRD4 activity mediates cat dander-induced inflammation and remodeling. Therapeutic modulation of the NF-κB-BRD4 pathway affects allergen-induced inflammation, epithelial cell-state changes, extracellular matrix production, and expansion of the subepithelial myofibroblast population.
Keywords: Bromodomain-containing protein 4; aeroallergen; airway remodeling; epigenetics; histone acetyltransferase activity; myofibroblast; nuclear factor κB.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Dahl R, Andersen PS, Chivato T, Valovirta E, de Monchy J. National prevalence of respiratory allergic disorders. Respir Med 2004; 98:398–403. - PubMed
-
- Prakash Ys, Halayko AJ, Gosens R, Panettieri RA Jr., Camoretti-Mercado B, Penn RB, et al. An Official American Thoracic Society Research Statement: Current Challenges Facing Research and Therapeutic Advances in Airway Remodeling. Am J Respir Crit Care Med 2017; 195:e4–e19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

