Epidermal growth factor receptor blockers for the treatment of ovarian cancer
- PMID: 30321910
- PMCID: PMC6430330
- DOI: 10.1002/14651858.CD007927.pub4
Epidermal growth factor receptor blockers for the treatment of ovarian cancer
Abstract
Background: This is an update of a previously published version of the review (Issue 10, 2011).Epithelial ovarian cancer (EOC) is the seventh most common cause of cancer death among women worldwide. Treatment consists of a combination of surgical debulking and platinum-based chemotherapy. Between 55% and 75% of women who respond to first-line therapy experience relapse within two years. Second-line chemotherapy is palliative and aims to reduce symptoms and prolong survival. Improved understanding about the molecular basis of EOC has led to the development of novel agents, such as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and anti-EGFR antibodies.
Objectives: To compare the effectiveness and harmful effects of interventions that target the epidermal growth factor receptor in the treatment of epithelial ovarian cancer (EOC).
Search methods: We searched the Cochrane Gynaecological Cancer Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL; 2010, Issue 4), MEDLINE, and Embase up to October 2010. We also searched registers of clinical trials, abstracts of scientific meetings, and reference lists of included studies, and we contacted experts in the field. This update includes further searches up to September 2017.
Selection criteria: Randomised controlled trials (RCTs) comparing anti-EGFR agents with or without conventional chemotherapy versus conventional chemotherapy alone or no treatment in women with histologically proven EOC.
Data collection and analysis: Two review authors independently abstracted data, assessed risk of bias, and performed GRADE assessment.
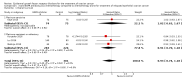
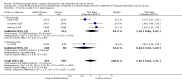
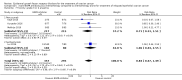
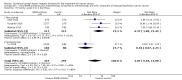
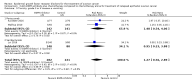
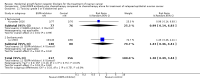
Main results: From 6105 references obtained through the literature search and an additional 15 references derived from grey literature searches, we identified seven RCTs that met our inclusion criteria and included 1725 participants. Trial results show that after first-line chemotherapy is provided, maintenance treatment with erlotinib (EGFR tyrosine kinase inhibitor (TKI)) probably makes little or no difference in overall survival (hazard ratio (HR) 0.99, 95% confidence interval (CI) 0.81 to 1.20; one study; 835 participants; low-certainty evidence) and may make little or no difference in progression-free survival (HR 1.05, 95% CI 0.90 to 1.23; one study; 835 participants; very low-certainty evidence). Less than 50% of participants provided quality of life data, and study authors reported these results incompletely. The certainty of evidence is very low, but treatment may reduce quality of life compared to observation.Treatment with an EGFR TKI (vandetanib) for women with relapsed EOC may make little or no difference in overall survival (HR 1.25, 95% CI 0.80 to 1.95; one study; 129 participants; low-certainty evidence) and may make little or no difference in progression-free survival (HR 0.99, 95% CI 0.69 to 1.42; one study; 129 participants; very low-certainty evidence). In treating patients with relapse, giving EGFR TKI may slightly increase some toxicities, such as severe rash (risk ratio (RR) 13.63, 95% CI 0.78 to 236.87; one study; 125 participants; very low-certainty evidence). Quality of life data were not available for meta-analysis.Anti-EGFR antibody treatment in relapsed EOC may or may not make a difference to overall survival (HR 0.93, 95% CI 0.74 to 1.18; four studies; 658 participants; moderate-certainty evidence) and may or may not have any effect on progression-free survival (HR 0.90, 95% CI 0.70 to 1.16; four studies; 658 participants; low-certainty evidence). Anti-EGFR antibody treatment may or may not increase side effects, including severe nausea and/or vomiting (RR 1.27, 95% CI 0.56 to 2.89; three studies; 503 participants; low-certainty evidence), severe fatigue (RR 1.06, 95% CI 0.66 to 1.73; I² = 0%; four studies; 652 participants; low-certainty evidence), and hypokalaemia (RR 2.01, 95% CI 0.80 to 5.06; I² = 0%; three studies; 522 participants; low-certainty evidence). Severe diarrhoea rates were heterogeneous across studies (RR 2.87, 95% CI 0.59 to 13.89; four studies; 652 participants; low-certainty evidence), and subgroup analysis revealed that severe diarrhoea was more likely with pertuzumab (RR 6.37, 95% CI 1.89 to 21.45; I² = 0%; three studies; 432 participants; low-certainty evidence) than with seribantumab treatment (RR 0.38, 95% CI 0.07 to 2.23; I² = 0%; one study; 220 participants; very low-certainty evidence). Quality of life data were incompletely reported, and we were unable to combine them in a meta-analysis.
Authors' conclusions: Current evidence suggests that an anti-EGFR single-agent biological treatment (EGFR TKI or anti-EGFR antibody) makes little or no difference to survival, either as maintenance treatment after first-line chemotherapy or in association with chemotherapy in recurrent cancer. Anti-EGFR therapy may increase some side effects and may or may not reduce quality of life.
Conflict of interest statement
Jo Morrison: I have no financial conflict of interest in the results of this review. Clemens Thoma: None known. Richard J Goodall: None known. Thomas J Lyons: None known. Kezia Gaitskell: None known. Alison J Wiggans: None known. Andrew Bryant: None known.
Figures





































Update of
-
Epidermal growth factor receptor blockers for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007927. doi: 10.1002/14651858.CD007927.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007927. doi: 10.1002/14651858.CD007927.pub4. PMID: 21975775 Updated.
References
References to studies included in this review
Chekerov 2017 {published data only}
-
- Chekerov R, Klare P, Krabisch P, Potenberg J, Heinrich G, Mueller L, et al. Panitumumab in platinum‐sensitive epithelial ovarian cancer patients with KRAS wild‐type: the PROVE‐study, a phase II randomized multicenter study of the North‐Eastern German Society of Gynaecologic Oncology. Journal of Clinical Oncology 2017;35(15):5558.
-
- WiSP Wissenschaftlicher Service Pharma GmbH. PROVE: A Randomized Phase II Trial of Standard Carboplatin‐based Chemotherapy With or Without Panitumumab in Platinum‐sensitive Recurrent Ovarian Cancer. ClinicalTrials.gov May 2011.
Coleman 2014 {published data only}
-
- Coleman RL, Moon J, Sood A, Branham D, Delmore JE, Bonebrake AJ, et al. Randomized phase II study of docetaxel plus vandetanib (D+V) versus docetaxel followed by vandetanib (D‐V) in patients with persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma (OC): SWOG S0904. European Journal of Cancer 2014; Vol. 50, issue 9:1638‐48. - PMC - PubMed
-
- Southwest Oncology Group, National Cancer Institute. Docetaxel with or without vandetanib in treating patients with persistent or recurrent ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer (NCT00872989). Protocol registered at ClinicalTrials.gov on 31 March 2009.
Kaye 2013 {published data only}
-
- Kaye SB, Poole CJ, Bidzinski M, Gianni L, Gorbunova V, Novikova E, et al. A randomized phase II study evaluating the combination of carboplatin‐based chemotherapy with pertuzumab versus carboplatin‐based therapy alone in patients with relapsed, platinum sensitive ovarian cancer. Journal of Clinical Oncology 2008;26(Suppl):Abstract 5520. - PubMed
-
- Kaye SB, Poole CJ, Danska‐Bidzinska A, Gianni L, Conte G, Gorbunova V, et al. A randomized phase II study evaluating the combination of carboplatin‐based chemotherapy with pertuzumab versus carboplatin‐based therapy alone in patients with relapsed, platinum‐sensitive ovarian cancer. Annals of Oncology 2013;14(1):145‐52. - PubMed
Kurzeder 2016 {published data only}
-
- Colombo N, Gonzalez‐Martin A, Oestergaard MZ, Rau J, Canzler U, Fabbro M, et al. Biomarker results from PENELOPE, a randomised phase III trial evaluating chemotherapy ± pertuzumab for platinum‐resistant ovarian cancer (PROC) with low tumour HER3 mRNA expression. International Journal of Gynecological Cancer 2015; Vol. 19th International Meeting of the European Society of Gynaecological Oncology, ESGO 2015, Nice, France:415‐7.
-
- Hilpert F, Greimel E, Ottevanger P, Rau J, Lorusso D, Kroep J, et al. Patient‐reported outcome (PRO) results from the AGO‐OVAR 2.20/ENGOT‐OV14/PENELOPE double‐blind placebo‐controlled randomised phase III trial evaluating chemotherapy ± pertuzumab for platinum‐resistant ovarian cancer (PROC). International Journal of Gynecological Cancer 2015; Vol. 19th International Meeting of the European Society of Gynaecological Oncology, ESGO 2015, Nice, France:465‐6.
-
- Hilpert F, Gropp‐Meier M, Schmalfeldt B, Rau J, Meier W, Hasenburg A, et al. Patient‐reported outcome (PRO) results from the AGO‐OVAR 2.20/ENGOT‐ov14/PENELOPE double‐blind placebo‐controlled randomised phase III trial evaluating chemotherapy ± pertuzumab for platinum‐resistant ovarian cancer (PROC). Oncology Research and Treatment 2018; Vol. 39:138.
-
- Hoffmann‐La Roche. A two‐part, randomized phase III, double‐blind, multicenter trial assessing the efficacy and safety of pertuzumab in combination with standard chemotherapy vs. placebo plus standard chemotherapy in women with recurrent platinum‐resistant epithelial ovarian cancer and low HER3 mRNA expression. Clinicaltrials.gov 2012.
-
- Kurzeder C, Bover I, Marme F, Rau J, Pautier P, Colombo N, et al. Double‐blind, placebo‐controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 MRNA‐expressing platinum‐resistant ovarian cancer (PENELOPE). Journal of Clinical Oncology 2016; Vol. 34, issue 21:2516‐25. - PubMed
Lui 2016 {published data only}
Makhija 2010 {published data only (unpublished sought but not used)}
-
- Glenn D, Ueland F, Bicher A, Dizon D, Gold M, Makhija S, et al. A randomized phase II trial with gemcitabine with or without pertuzumab (rhuMAb 2C4) in platinum‐resistant ovarian cancer (OC): preliminary safety data [abstract]. Journal of Clinical Oncology; 2006 ASCO Annual Meeting Proceedings Part I, 2006;24(Suppl):13001.
-
- Lalla D, Paton V, Lin CY, Dizon DS. Affecting quality of life in ovarian cancer: encouraging results from a randomized phase II trial of gemcitabine ± pertuzumab in women with platinum‐resistant disease [Abstract]. Journal of Clinical Oncology 2008;26(Suppl):Abstract 5550.
-
- Makhija S, Amler LC, Glenn D, Ueland FE, Gold MA, Dizon DS, et al. Clinical activity of gemcitabine plus pertuzumab in platinum‐resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Journal of Clinical Oncology 2010;28(7):1215‐23. - PubMed
-
- Makhija S, Glenn D, Ueland F, Gold M, Dizon D, Paton V, et al. Results from a phase II randomized, placebo‐controlled, double‐blind trial suggest improved PFS with the addition of pertuzumab to gemcitabine in patients with platinum‐resistant ovarian, fallopian tube or primary peritoneal cancer. Journal of Clinical Oncology 2007;25(Suppl):Abstract 5507.
Vergote 2014 {published data only}
-
- European Organization for Research and Treatment of Cancer. Erlotinib or observation in treating patients who have undergone first‐line chemotherapy for ovarian cancer, peritoneal cancer, or fallopian tube cancer (MRC OV07). Protocol registered at ClinicalTrials.gov on 7 December 2005.
-
- Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first‐line platin‐based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer‐Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. Journal of Clinical Oncology 2014; Vol. 32, issue 4:320‐6. - PubMed
References to studies excluded from this review
Annunziata 2010 {published data only}
Bauman 2012 {published data only}
-
- Bauman J, Verschraegen C, Belinsky S, Muller C, Rutledge T, Fekrazad M, et al. A phase I study of 5‐azacytidine and erlotinib in advanced solid tumor malignancies. Cancer Chemotherapy and Pharmacology 2012; Vol. 69, issue 2:547‐54. - PubMed
Blank 2010 {published data only}
-
- National Cancer Institute. A Phase II Study of OSI 774 In Combination With Carboplatin and Paclitaxel in Patients With Ovarian, Cancer of the Fallopian Tube or Primary Peritoneal Carcinoma. ClinicalTrials.gov identifier: NCT00059787. Protocol registered at ClinicalTrials.gov on 6 May 2003.
Bookman 2003 {published data only}
-
- Bookman MA, Darcy KM, Clarke‐Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti‐HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. Journal of Clinical Oncology 2003;21:283‐90. - PubMed
Campos 2005 {published data only}
-
- Campos S, Hamid O, Seiden MV, Oza A, Plante M, Potkul RK, et al. Multicenter, randomized phase II trial of oral CI‐1033 for previously treated advanced ovarian cancer. Journal of Clinical Oncology 2005;23:5597‐604. - PubMed
Campos 2010 {published data only}
-
- Campos SM, Berlin ST, Parker LM, Chen WY, Bunnell CA, Atkinson T, et al. Phase I trial of liposomal doxorubicin and ZD1839 in patients with refractory gynecological malignancies or metastatic breast cancer. International Journal of Clinical Oncology 2010; Vol. 15, issue 4:390‐8. - PubMed
Campos 2011 (NCT00520013) {published data only}
-
- Campos A, Atkinson T, Berlin A. STAC: a phase II study of carboplatin/paclitaxel/bevacizumab followed by randomization to either bevacizumab alone or erlotinib and bevacizumab in the upfront management of patients with ovarian, fallopian tube or peritoneal cancer [Meeting abstract]. Gynecologic Oncology 2011;120(Suppl 1):S79. Abstract 181.
-
- Dana‐Farber Cancer Institute. Avastin and Erlotinib as First Line Consolidation Chemotherapy After Carboplatin, Paclitaxel, and Avastin (CTA) Induction Therapy for Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer, and Papillary Serous Mullerian Tumors. ClinicalTrials.gov Identifier: NCT00520013. Protocol registered at ClinicalTrials.gov on 22 August 2007.
Chambers 2010 {published data only}
-
- Chambers SK, Clouser MC, Baker AF, Roe DJ, Cui H, Brewer MA, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clinical Cancer Research 2010;16(21):5320‐8. - PMC - PubMed
-
- University of Arizona, Genentech. Phase II Open‐Label Trial of Erlotinib (Tarceva) and Bevacizumab in Women With Advanced Ovarian Cancer. ClinicalTrials.gov Identifier: NCT00130520. Protocol registered at ClinicalTrials.gov on 12 August 2005.
Ciunci 2014 {published data only}
Dinh 2008 {published data only}
-
- Dinh P, Harnett P, Piccart‐Gebhart MJ, Awada A. New therapies for ovarian cancer: cytotoxics and molecularly targeted agents. Critical Reviews in Oncology/Hematology 2008;67:103‐12. - PubMed
Galsky 2012 {published data only}
-
- Galsky MD, Hoff DD, Neubauer M, Anderson T, Fleming M, Nagarwala Y, et al. Target‐specific, histology‐independent, randomized discontinuation study of lapatinib in patients with HER2‐amplified solid tumors. Investigational New Drugs 2012; Vol. 30, issue 2:695‐701. - PubMed
Garcia 2012 {published data only}
-
- Garcia AA, Sill MW, Lankes HA, Godwin AK, Mannel RS, Armstrong DK, et al. A phase II evaluation of lapatinib in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology 2012; Vol. 124, issue 3:569‐74. - PMC - PubMed
-
- Gynecologic Oncology Group, National Cancer Institute. A Phase II Evaluation of Lapatinib (GW572016) (NCI‐Supplied Agent, NSC #727989) in the Treatment of Persistent or Recurrent Epithelial Ovarian or Primary Peritoneal Carcinoma. ClinicalTrials.gov Identifier: NCT00113373. Protocol registered at ClinicalTrials.gov on 7 June 2005.
Gordon 2005 {published data only}
-
- Gordon AN, Finkler N, Edwards P, Garcia AA, Crozier M, Irwin DH, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. International Journal of Gynecological Cancer 2005;15:785‐92. - PubMed
Gordon 2006 {published data only}
-
- Genentech Inc. A Phase II, Open‐label, Multicenter Study to Evaluate the Effect of Tumor‐based HER2 Activation on the Efficacy of rhuMAb 2C4 (Pertuzumab) in Subjects With Advanced, Refractory or Recurrent Ovarian Cancer. ClinicalTrials.gov identifier: NCT00058552. Protocol registered at ClinicalTrials.gov on 8 April 2003.
-
- Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. Journal of Clinical Oncology 2006;24:4324‐32. - PubMed
Guastalla 2007 {published data only}
-
- Guastalla JP, Allouache D, Combe M, Weber B, Cretin J, Cure H, et al. HER2 overexpression and amplification in advanced ovarian cancer (AOC): treatment with trustuzumab ‐ a GINECO study. Journal of Clinical Oncology 2007;25(18 Suppl):5559.
Hariprasad 2006 {published data only}
-
- Hariprasad R, Kumar L, Patnaik R, Gupta A, Kumar S. Maintenance therapy in epithelial ovarian cancer: could EGFR‐inhibitor gefitinib be a candidate drug? A pilot study. Journal of Clinical Oncology 2006;24(18 Suppl):15046.
Hariprasad 2009 {published data only}
-
- Hariprasad R, Kumar L, Kumar S, Bhatla N, Thulkar S, Das P. Advanced epithelial ovarian cancer (EOC): is there a place for maintenance therapy in patients with sub‐optimal debulking?. Journal of Clinical Oncology 2009;27(Suppl):abstr e14580.
Harter 2013 {published data only}
-
- Harter P, Sehouli J, Kimmig R, Rau J, Hilpert F, Kurzeder C, et al. Addition of vandetanib to pegylated liposomal doxorubicin (PLD) in patients with recurrent ovarian cancer. A randomized phase I/II study of the AGO Study Group (AGO‐OVAR 2.13). Investigational New Drugs 2013;31(6):1499‐504. [DOI] - PubMed
Hirte 2010 {published data only}
-
- Hirte H, Oza A, Hoskins P, Ellard S, Grimshaw R, Dubuc‐Lissoir J, et al. Phase II study of OSI‐774 given in combination with carboplatin in patients (pts) with recurrent epithelial ovarian cancer (EOC): NCIC CTG IND.149. European Journal of Cancer 2003;1(5 Suppl: ECCO 12 Abstract Book):A‐159, S51.
-
- Hirte H, Oza A, Swenerton K, Ellard SL, Grimshaw R, Fisher B, et al. A phase II study of erlotinib (OSI‐774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer. Gynecologic Oncology 2010;118(3):308‐12. - PubMed
-
- NCIC Clinical Trials Group. A Phase II Study of OSI‐774 (NSC 718781) Given in Combination With Carboplatin in Patients With Recurrent Epithelial Ovarian Cancer. ClinicalTrials.gov identifier: NCT00030446. Protocol registered at ClinicalTrials.gov on 14 February 2002.
Jhaveri 2012 {published data only}
-
- Jhaveri K, Miller K, Rosen L, Schneider B, Chap L, Hannah A, et al. A phase I dose‐escalation trial of trastuzumab and alvespimycin hydrochloride (KOS‐1022; 17 DMAG) in the treatment of advanced solid tumors. Clinical Cancer Research 2012; Vol. 18, issue 18:5090‐8. - PubMed
Joly 2009 {published data only}
-
- Jolly F, Weber B, Pautier P, Fabbro M, Selle F, Krieger S. Combined topotecan and lapatinib in patients with early recurrent ovarian or peritoneal cancer after first line of platinum based chemotherapy: a French FEDEGYN‐FNCLCC phase II trial. Journal of Clinical Oncology 2009;27(15S):Abstract 5555.
Kimball 2008 {published data only}
-
- Kimball KJ, Numnum TM, Kirby TO, Zamboni WC, Estes JM, Barnes MN, et al. A phase I study of lapatinib in combination with carboplatin in women with platinum sensitive recurrent ovarian carcinoma. Gynecologic Oncology 2008;111:95‐101. - PubMed
Konner 2008 {published data only}
-
- ImClone LLC, Bristol‐Myers Squibb. A Phase II Study of Cetuximab (C225)/Paclitaxel/Carboplatin for the Initial Treatment of Advanced Stage Ovarian, Primary Peritoneal, and Fallopian Tube Cancer. ClinicalTrials.gov Identifier: NCT00063401. Protocol registered at ClinicalTrials.gov on 25 June 2003.
-
- Konner J, Schilder RJ, DeRosa FA, Gerst SR, Tew WP, Sabbatini PJ, et al. A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced‐stage ovarian, primary peritoneal or fallopian tube cancer. Gynecologic Oncology 2008;110:140‐5. - PubMed
Koolen 2011 {published data only}
-
- Koolen SLW, Witteveen PO, Jansen RS, Langenberg MH, Kronemeijer RH, Nol A, et al. Phase I study of oral gemcitabine prodrug (LY2334737) alone and in combination with erlotinib in patients with advanced solid tumors. Clinical Cancer Research 2011; Vol. 17, issue 18:6071‐82. - PubMed
Krasner 2005 {published data only}
-
- A Phase II Study of ZD1839 (Iressa) Plus Anastrozole (Arimidex) in Patients With Relapsed Ovarian Cancer. ClinicalTrials.gov Identifier: NCT00181688. Protocol registered at ClinicalTrials.gov on 12 September 2005.
-
- Krasner CN, Debernardo RL, Findley M, Penson R, Matulonis U, Atkinson T, et al. Phase II trial of anastrozole in combination with gefitinib in women with asymptomatic mullerian cancer. Journal of Clinical Oncology 2005;23:Abstract 5063.
Lheureux 2012 {published data only}
-
- Lheureux S, Krieger S, Weber B, Pautier P, Fabbro M, Selle F, et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. International Journal of Gynecological Cancer 2012; Vol. 22, issue 9:1483‐8. - PubMed
NCT00861120 {unpublished data only}
-
- Jakobsen A, Steffensen KD. Panitumumab and Pegylated Liposomal Doxorubicin for Platinum‐Resistant Epithelial Ovarian Cancer With KRAS Wild‐type (PaLiDo). ClinicalTrials.gov 2012. - PubMed
NCT01296035 {unpublished data only}
-
- McCourt C. A Phase II Evaluation of Panitumumab and Gemcitabine as Treatment for Women With Recurrent Epithelial Ovarian Cancer. ClinicalTrials.gov 2011.
Nimeiri 2008 {published data only}
-
- National Cancer Institute. A Phase II Trial of Bevacizumab (NSC# 704865) and OSI‐774 (NSC# 718781) in Recurrent Ovarian Cancer, Fallopian Tube Carcinoma or Primary Peritoneal Carcinoma. ClinicalTrials.gov Identifier: NCT00126542. Protocol registered at ClinicalTrials.gov on 2 August 2005.
-
- Nimeiri HS, Oza AM, Morgan RJ, Friberg G, Kasza K, Faoro L, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecologic Oncology 2008;110(1):49‐55. - PMC - PubMed
Palayekar 2008 {published data only}
-
- Palayekar MJ, Herzog TJ. The emerging role of epithelial growth factor receptor inhibitors in ovarian cancer. International Journal of Gynecological Cancer 2008;18(5):879‐90. - PubMed
Pautier 2010 {published data only}
-
- Pautier P, Joly F, Kerbrat P, Bougnoux P, Fumoleau P, Petit T, et al. Phase II study of gefitinib in combination with paclitaxel and carboplatin as second‐line therapy for ovarian, tubal or peritoneal adenocarcinoma (1839IL/0074). Gynecologic Oncology 2010;116:157‐62. - PubMed
Posadas 2007 {published data only}
Ray‐Coquard 2008 {published data only}
-
- Ray‐Coquard I, Guastalla JP, Allouache D, Combe M, Weber B, Cretin J, et al. HER2 overexpression/amplification and trastuzumab treatment in advanced ovarian cancer: a GINECO phase II study. Clinical Ovarian Cancer 2008;1(1):54‐9.
Schilder 2005 {published data only}
-
- Gynecologic Oncology Group, National Cancer Institute. A Phase II Trial of ZD1839 (Iressa) (NSC# 715055) in the Treatment of Persistent or Recurrent Epithelial Ovarian or Primary Peritoneal Carcinoma. ClinicalTrials.gov Identifier: NCT00023699. Protocol registered at ClinicalTrials.gov on 13 September 2001.
-
- Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of EGFR mutations and immunohistochemical expression: a gynecologic oncology group study. Clinical Cancer Research 2005;11(15):5539‐48. - PubMed
Schilder 2009 {published data only}
-
- ImClone LLC, Bristol‐Myers Squibb. A Phase II Trial of Single‐agent Cetuximab Dose Escalated to Rash in Patients With Persistent or Recurrent Epithelial Ovarian or Primary Peritoneal Carcinoma. ClinicalTrials.gov Identifier: NCT00082212. Protocol registered at ClinicalTrials.gov on 3 May 2004.
-
- Schilder RJ, Pathak HB, Lokshin AE, Holloway RW, Alvarez RD, Aghajanian C, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecologic Oncology 2009;113(1):21‐7. - PMC - PubMed
Secord 2008 {published data only}
-
- Gynecologic Oncology Group. A Phase II Evaluation of Cetuximab (C225, NSC #714692) in Combination With Carboplatin (NSC #241240) in the Treatment of Recurrent Platinum‐Sensitive Ovarian or Primary Peritoneal Cancer. ClinicalTrials.gov Identifier: NCT00086892. Protocol registered at ClinicalTrials.gov on 8 July 2004.
-
- Secord AA, Blessing JA, Armstrong DK, Rodgers WH, Miner Z, Barnes MN, et al. A phase II trial of cetuximab and carboplatin in relapsed platinum‐sensitive ovarian cancer and evaluation of epidural growth factor receptor expression: a gynecologic oncology group study. Gynecologic Oncology 2008;108(3):493‐4. - PMC - PubMed
Seiden 2007 {published data only}
-
- EMD Serono. An Open‐Label Phase II Study in Subjects With Recurrent EGFR‐Positive Ovarian Cancer to Investigate the Safety and Efficacy of EMD 72000 Administered as a Single Agent. ClinicalTrials.gov Identifier: NCT00073541. Protocol registered at ClinicalTrials.gov on 24 November 2003.
-
- Seiden MV, Burris HA, Matulonis U, Hall JB, Armstrong DK, Speyer J, et al. A phase II trial of EMD72000 (matuzumab), a humanised anti‐EGFR monoclonal antibody, in patients with platinum‐resistant ovarian and primary peritoneal malignancies. Gynecologic Oncology 2007;104(3):727‐31. - PubMed
Steffensen 2013 {published data only}
-
- Steffensen KD, Waldstrøm M, Lund B, Bergfeldt K, Keldsen N, Marth C, et al. Panitumumab and pegylated liposomal doxorubicin to platinum‐resistant epithelial ovarian cancer with KRAS wild‐type: an ongoing, nonrandomized, multicenter, phase II trial. Journal of Clinical Oncology 2010;28(15 Suppl):abstr TPS254.
-
- Steffensen KD, Waldstrøm M, Pallisgard N, Lund B, Bergfeldt K, Wihl J, et al. Panitumumab and pegylated liposomal doxorubicin in platinum‐resistant epithelial ovarian cancer with KRAS wild‐type: the PaLiDo study, a phase II nonrandomized multicenter study. International Journal of Gynecological Cancer 2013;23(1):73‐80. - PubMed
Vasey 2008 {published data only}
Vlahovic 2012 {published data only}
Wagner 2007 {published data only}
-
- Wagner U, du Bois A, Pfistrerer J, Houber J, Loibl S, Luck HJ, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum‐taxane based therapy ‐ a phase II trial of the AGO ovarian cancer study group (AGO‐OVAR 2.6). Gynecologic Oncology 2007;105(1):132‐7. - PubMed
Weroha 2011 {published data only}
Additional references
Bandera 2003
-
- Bandera CA, Tsui HW, Mok SC, Tsui FW. Expression of cytokines and receptors in normal, immortalized, and malignant ovarian epithelial cell lines. Anticancer Research 2003;23(4):3151‐7. - PubMed
Bartlett 1996
Baselga 2001
-
- Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Seminars in Oncology 2001;28(5 Suppl 16):4‐11. - PubMed
Baselga 2002
-
- Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. Journal of Clinical Oncology 2002;20(21):4292‐302. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. - PubMed
Ciardiello 1999
-
- Ciardiello F, Bianco R, Damiano V, Lorenzo S, Pepe S, Placido S, et al. Antitumor activity of sequential treatment with topotecan and anti‐epidermal growth factor receptor monoclonal antibody C225. Clinical Cancer Research 1999;5(4):909‐16. - PubMed
Ciardiello 2001
-
- Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clinical Cancer Research 2001;7(5):1459‐65. - PubMed
Cooley 1999
-
- Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody‐dependent cellular cytotoxicity against LFA‐3 and HER2/neu. Experimental Hematology 1999;27(10):1533‐41. - PubMed
Covidence
-
- Covidence systematic review software, Veritas Health Innovation. www.covidence.org. Melbourne, Australia.
CTEP 2017
-
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs... 2017.
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Downward 1984
-
- Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, et al. Close similarity of epidermal growth factor receptor and v‐erb‐B oncogene protein sequences. Nature 1984;307(5951):521‐7. - PubMed
Ekstrand 1992
-
- Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N‐ and/or C‐terminal tails. Proceedings of the National Academy of Science USA 1992;89(10):4309‐13. - PMC - PubMed
Erickson 2013
Giesinger 2016
Glaysher 2013
GLOBOCAN 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase. Lyon: IARCPress, 2013, issue No. 11 [Internet].
Goldstein 1995
-
- Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clinical Cancer Research 1995;1(11):1311‐8. - PubMed
GRADE Working Group
GRADEpro GDT
-
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Hamilton (ON): McMaster University, 2015. Developed by Evidence Prime, Inc.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Montori M, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. - PubMed
Higgins 2003
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. Cancer Journal for Clinicians 2008;58(2):71‐96. - PubMed
Jensen 2011
Kehoe 2015
-
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open‐label, randomised, controlled, non‐inferiority trial. The Lancet 2015;386(9990):249‐57. - PubMed
Morrison 2012
Moscatello 1995
-
- Moscatello DK, Holgado‐Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Research 1995;55(23):5536‐9. - PubMed
Moulder 2001
-
- Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)‐over expressing breast cancer cells in vitro and in vivo. Cancer Research 2001;61(24):8887‐95. - PubMed
NCT00263822
-
- NCT00263822. A Randomized, Multicenter, Phase III Study of Erlotinib versus Observation in Patients With No Evidence of Disease Progression After First Line, Platinum‐Based Chemotherapy for High‐Risk Ovarian Epithelial, Primary Peritoneal, or Fallopian Tube Cancer. MetaRegister of Controlled Trials 2005.
NCT00520013
-
- NCT00520013. A Randomized Phase II Trial of Avastin (A) or Avastin and Erlotinib (AE) as First Line Consolidation Chemotherapy After Carboplatin, Paclitaxel and Avastin (CTA) Induction Therapy for Newly Diagnosed Advanced Ovarian, Fallopian Tube and Primary Peritoneal Cancer & Papillary Serous Mullerian Tumors. MetaRegister of Controlled Trials 2007.
Nicholson 2001
-
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. European Journal of Cancer 2001;37(Suppl 4):S9‐15. - PubMed
Okamoto 2018
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Quinn 2001
-
- Quinn M, Babb B, Brock A, Jones J. In: Office for National Statistics, editor(s). Cancer Trends in England and Wales. London: The Stationery Office, 2001.
Schünemann 2014
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Shepherd 1989
-
- Shepherd JH. Revised FIGO staging for gynaecological cancer. British Journal of Obstetrics and Gynaecology 1989;96(8):889‐92. - PubMed
Slamon 1989
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER‐2/neu proto‐oncogene in human breast and ovarian cancer. Science 1989;244(4905):707‐12. - PubMed
Stewart 1999
Teplinsky 2015
-
- Teplinsky E, Muggia F. EGFR and HER2: is there a role in ovarian cancer?. Translational Cancer Research 2015;4(1):107‐17.
Vergote 2010
-
- Vergote I, Tropé CG, Amant F, Kristensen GB, Sardi JE, Ehlen T, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine 2010;363(10):943‐53. - PubMed
Wedge 2002
-
- Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Research 2002;62(16):4645‐55. - PubMed
References to other published versions of this review
Gaitskell 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

