Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel
- PMID: 30322014
- PMCID: PMC6213782
- DOI: 10.3390/ijms19103132
Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel
Abstract
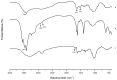
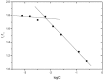
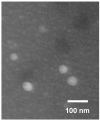
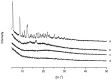
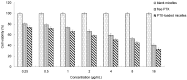
The present investigation aimed to develop a tumor-targeting drug delivery system for paclitaxel (PTX). The hydrophobic deoxycholic acid (DA) and active targeting ligand folic acid (FA) were used to modify water-soluble chitosan (CS). As an amphiphilic polymer, the conjugate FA-CS-DA was synthesized and characterized by Proton nuclear magnetic resonance (¹H-NMR) and Fourier-transform infrared spectroscopy (FTIR) analysis. The degree of substitutions of DA and FA were calculated as 15.8% and 8.0%, respectively. In aqueous medium, the conjugate could self-assemble into micelles with the critical micelle concentration of 6.6 × 10-3 mg/mL. Under a transmission electron microscope (TEM), the PTX-loaded micelles exhibited a spherical shape. The particle size determined by dynamic light scattering was 126 nm, and the zeta potential was +19.3 mV. The drug loading efficiency and entrapment efficiency were 9.1% and 81.2%, respectively. X-Ray Diffraction (XRD) analysis showed that the PTX was encapsulated in the micelles in a molecular or amorphous state. In vitro and in vivo antitumor evaluations demonstrated the excellent antitumor activity of PTX-loaded micelles. It was suggested that FA-CS-DA was a safe and effective carrier for the intravenous delivery of paclitaxel.
Keywords: amphiphilic polymer; chitosan; deoxycholic acid; folic acid; micelles; paclitaxel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Tissue distribution and pharmacokinetics evaluation of DOMC-FA micelles for intravenous delivery of PTX.J Drug Target. 2013 Feb;21(2):137-45. doi: 10.3109/1061186X.2012.731067. Epub 2012 Oct 9. J Drug Target. 2013. PMID: 23046502
-
Polymeric Micelles Based on Modified Glycol Chitosan for Paclitaxel Delivery: Preparation, Characterization and Evaluation.Int J Mol Sci. 2018 May 23;19(6):1550. doi: 10.3390/ijms19061550. Int J Mol Sci. 2018. PMID: 29882845 Free PMC article.
-
Tumor-targeting micelles based on folic acid and α-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery.Colloids Surf B Biointerfaces. 2019 May 1;177:11-18. doi: 10.1016/j.colsurfb.2019.01.044. Epub 2019 Jan 23. Colloids Surf B Biointerfaces. 2019. PMID: 30690425
-
α-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: preparation, characterization and in vitro/in vivo evaluations.Int J Pharm. 2012 Feb 28;423(2):480-8. doi: 10.1016/j.ijpharm.2011.12.004. Epub 2011 Dec 13. Int J Pharm. 2012. PMID: 22183133
-
Development and in vitro/in vivo evaluation of a novel targeted polymeric micelle for delivery of paclitaxel.Int J Biol Macromol. 2015 Sep;80:29-40. doi: 10.1016/j.ijbiomac.2015.05.062. Epub 2015 Jun 18. Int J Biol Macromol. 2015. PMID: 26093319
Cited by
-
Preparation, Characterization, and Antioxidant Properties of Self-Assembled Nanomicelles of Curcumin-Loaded Amphiphilic Modified Chitosan.Molecules. 2024 Jun 6;29(11):2693. doi: 10.3390/molecules29112693. Molecules. 2024. PMID: 38893567 Free PMC article.
-
Nano-Sized Fucoidan Interpolyelectrolyte Complexes: Recent Advances in Design and Prospects for Biomedical Applications.Int J Mol Sci. 2023 Jan 30;24(3):2615. doi: 10.3390/ijms24032615. Int J Mol Sci. 2023. PMID: 36768936 Free PMC article. Review.
-
Targeting Strategies for Enhancing Paclitaxel Specificity in Chemotherapy.Front Cell Dev Biol. 2021 Mar 29;9:626910. doi: 10.3389/fcell.2021.626910. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33855017 Free PMC article. Review.
-
Exploiting Apical Sodium-Dependent Bile Acid Transporter (ASBT)-Mediated Endocytosis with Multi-Functional Deoxycholic Acid Grafted Alginate Amide Nanoparticles as an Oral Insulin Delivery System.Pharm Res. 2024 Feb;41(2):335-353. doi: 10.1007/s11095-023-03641-7. Epub 2023 Dec 19. Pharm Res. 2024. PMID: 38114803
-
Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer.Cancers (Basel). 2023 Feb 6;15(4):1023. doi: 10.3390/cancers15041023. Cancers (Basel). 2023. PMID: 36831369 Free PMC article. Review.
References
-
- Eloy J.O., Petrilli R., Topan J.F., Antonio H.M.R., Barcellos J.P.A., Chesca D.L., Serafini L.N., Tiezzi D.G., Lee R.J., Marchetti J.M. Co-loaded paclitaxel/rapamycin liposomes: Development, characterization and in vitro and in vivo evaluation for breast cancer therapy. Colloids Surf. B Biointerfaces. 2016;141:74–82. doi: 10.1016/j.colsurfb.2016.01.032. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

