Use of Plasma-Synthesized Nano-Catalysts for CO Hydrogenation in Low-Temperature Fischer⁻Tropsch Synthesis: Effect of Catalyst Pre-Treatment
- PMID: 30322025
- PMCID: PMC6215254
- DOI: 10.3390/nano8100822
Use of Plasma-Synthesized Nano-Catalysts for CO Hydrogenation in Low-Temperature Fischer⁻Tropsch Synthesis: Effect of Catalyst Pre-Treatment
Abstract
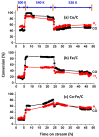
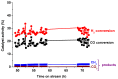
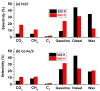
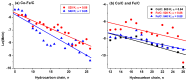
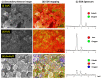
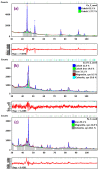
A study was done on the effect of temperature and catalyst pre-treatment on CO hydrogenation over plasma-synthesized catalysts during the Fischer⁻Tropsch synthesis (FTS). Nanometric Co/C, Fe/C, and 50%Co-50%Fe/C catalysts with BET specific surface area of ~80 m² g⁻1 were tested at a 2 MPa pressure and a gas hourly space velocity (GHSV) of 2000 cm³ h-1 g-1 of a catalyst (at STP) in hydrogen-rich FTS feed gas (H₂:CO = 2.2). After pre-treatment in both H₂ and CO, transmission electron microscopy (TEM) showed that the used catalysts shifted from a mono-modal particle-size distribution (mean ~11 nm) to a multi-modal distribution with a substantial increase in the smaller nanoparticles (~5 nm), which was statistically significant. Further characterization was conducted by scanning electron microscopy (SEM with EDX elemental mapping), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The average CO conversion at 500 K was 18% (Co/C), 17% (Fe/C), and 16% (Co-Fe/C); 46%, 37%, and 57% at 520 K; and 85%, 86% and 71% at 540 K respectively. The selectivity of Co/C for C5+ was ~98% with 8% gasoline, 61%, diesel and 28% wax (fractions) at 500 K; 22% gasoline, 50% diesel, and 19% wax at 520 K; and 24% gasoline, 34% diesel, and 11% wax at 540 K, besides CO₂ and CH₄ as by-products. Fe-containing catalysts manifested similar trends, with a poor conformity to the Anderson⁻Schulz⁻Flory (ASF) product distribution.
Keywords: CO-hydrogenation; low-temperature Fischer–Tropsch; nano-catalysts; plasma synthesis; pre-treatment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures













References
-
- Sun X., Suarez A.I.O., Meijerink M., van Deelen T., Ould-Chikh S., Zečević J., de Jong K.P., Kapteijn F., Gascon J. Manufacture of highly loaded silica-supported cobalt Fischer–Tropsch catalysts from a metal organic framework. Nat. Commun. 2017;8:1680. doi: 10.1038/s41467-017-01910-9. - DOI - PMC - PubMed
-
- Zaporotskova I.V., Boroznina N.P., Parkhomenko Y.N., Kozhitov L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016;2:95–105. doi: 10.1016/j.moem.2017.02.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

