Effects of Panax Notoginseng Saponins on Esterases Responsible for Aspirin Hydrolysis In Vitro
- PMID: 30322078
- PMCID: PMC6213075
- DOI: 10.3390/ijms19103144
Effects of Panax Notoginseng Saponins on Esterases Responsible for Aspirin Hydrolysis In Vitro
Abstract
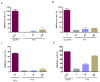
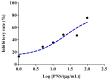
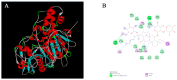
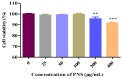
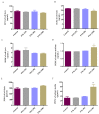
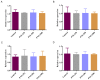
Herb⁻drug interactions strongly challenge the clinical combined application of herbs and drugs. Herbal products consist of complex pharmacological-active ingredients and perturb the activity of drug-metabolizing enzymes. Panax notoginseng saponins (PNS)-based drugs are often combined with aspirin in vascular disease treatment in China. PNS was found to exhibit inhibitory effects on aspirin hydrolysis using Caco-2 cell monolayers. In the present study, a total of 22 components of PNS were separated and identified by UPLC-MS/MS. Using highly selective probe substrate analysis, PNS exerted robust inhibitory potency on human carboxylesterase 2 (hCE2), while had a minor influence on hCE1, butyrylcholinesterase (BChE) and paraoxonase (PON). These effects were also verified through molecular docking analysis. PNS showed a concentration-dependent inhibitory effect on hydrolytic activity of aspirin in HepaRG cells. The protein level of hCE2 in HepaRG cells was suppressed after PNS treatment, while the level of BChE or PON1 in the extracellular matrix were elevated after PNS treatment. Insignificant effect was observed on the mRNA expression of the esterases. These findings are important to understand the underlying efficacy and safety of co-administration of PNS and aspirin in clinical practice.
Keywords: HepaRG cells; Panax notoginseng saponins; aspirin; herb–drug interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Inhibitory Influence of Panax notoginseng Saponins on Aspirin Hydrolysis in Human Intestinal Caco-2 Cells.Molecules. 2018 Feb 18;23(2):455. doi: 10.3390/molecules23020455. Molecules. 2018. PMID: 29463025 Free PMC article.
-
Effect of Panax notoginseng saponins on the pharmacokinetics of aspirin in rats.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Jan 1;1040:136-143. doi: 10.1016/j.jchromb.2016.12.007. Epub 2016 Dec 6. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 27978468
-
Effect of aspirin on the pharmacokinetics and absorption of panax notoginseng saponins.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Feb 1;1074-1075:25-33. doi: 10.1016/j.jchromb.2017.12.033. Epub 2018 Jan 2. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 29329092
-
Anti-diabetic potential of Panax notoginseng saponins (PNS): a review.Phytother Res. 2014 Apr;28(4):510-6. doi: 10.1002/ptr.5026. Epub 2013 Jul 11. Phytother Res. 2014. PMID: 23846979 Review.
-
[Research progress on regulation effect of Panax notoginseng saponins on mitochondria].Zhongguo Zhong Yao Za Zhi. 2017 Mar;42(5):870-874. doi: 10.19540/j.cnki.cjcmm.20170121.010. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 28994528 Review. Chinese.
Cited by
-
Marine-Derived Bioactive Peptides Self-Assembled Multifunctional Materials: Antioxidant and Wound Healing.Antioxidants (Basel). 2023 May 31;12(6):1190. doi: 10.3390/antiox12061190. Antioxidants (Basel). 2023. PMID: 37371920 Free PMC article.
-
Comparative Analysis of Compatibility Influence on Invigorating Blood Circulation for Combined Use of Panax Notoginseng Saponins and Aspirin Using Metabolomics Approach.Front Pharmacol. 2021 Apr 30;12:544002. doi: 10.3389/fphar.2021.544002. eCollection 2021. Front Pharmacol. 2021. PMID: 33995000 Free PMC article.
References
-
- Murray J., Picking D., Lamm A., McKenzie J., Hartley S., Watson C., Williams L., Lowe H., Delgoda R. Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions. Fitoterapia. 2016;111:138–146. doi: 10.1016/j.fitote.2016.04.011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

