NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients
- PMID: 30322396
- PMCID: PMC6190561
- DOI: 10.1186/s13058-018-1049-0
NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients
Abstract
Background: The presence of disseminated tumor cells (DTCs) in bone marrow (BM) is an independent prognostic factor in early breast cancer but does not uniformly predict outcome. Tumor cells can persist in a quiescent state over time, but clinical studies of markers predicting the awakening potential of DTCs are lacking. Recently, experiments have shown that NR2F1 (COUP-TF1) plays a key role in dormancy signaling.
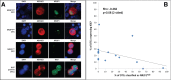
Methods: We analyzed the NR2F1 expression in DTCs by double immunofluorescence (DIF) staining of extra cytospins prepared from 114 BM samples from 86 selected DTC-positive breast cancer patients. Samples collected at two or more time points were available for 24 patients. Fifteen samples were also analyzed for the proliferation marker Ki67.
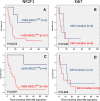
Results: Of the patients with detectable DTCs by DIF, 27% had ≥ 50% NR2F1high DTCs, chosen a priori as the cut-off for "dormant profile" classification. All patients with systemic relapse within 12 months after BM aspiration carried ≤ 1% NR2F1high DTCs, including patients who transitioned from having NR2F1high-expressing DTCs in previous BM samples. Of the patients with serial samples, half of those with no relapse at follow-up had ≥ 50% NR2F1high DTCs in the last BM aspiration analyzed. Among the 18 relapse-free patients at the time of the last DTC-positive BM aspiration with no subsequent BM analysis performed, distant disease-free intervals were favorable for patients carrying ≥ 50% NR2F1high DTCs compared with those with predominantly NR2F1low DTCs (p = 0.007, log-rank). No survival difference was observed by classification according to Ki67-expressing DTCs (p = 0.520).
Conclusions: Our study translates findings from basic biological analysis of DTC dormancy to the clinical situation and supports further clinical studies of NR2F1 as a marker of dormancy.
Keywords: Bone marrow; Breast cancer; DTC; Disseminated tumor cells; Dormancy; Micrometastasis; NR2F1; Occult disease.
Conflict of interest statement
Ethics approval and consent to participate
NeoTax Study: Norwegian Regional Ethics Committee approval number 273/96–82.96 REK Helseregion III. Oslo1 Study: Norwegian Regional Ethics Committee approval number S-97103. SATT Study: Norwegian Regional Ethics Committee approval number S-03032. Informed consent to participate was given by all patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Juckstock J, Borgen E, Rack B, Braun S, Sommer H, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17(9):2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. - DOI - PubMed
-
- Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69(14):5664–5672. doi: 10.1158/0008-5472.CAN-08-3820. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

