Registration of published randomized trials: a systematic review and meta-analysis
- PMID: 30322399
- PMCID: PMC6190546
- DOI: 10.1186/s12916-018-1168-6
Registration of published randomized trials: a systematic review and meta-analysis
Abstract
Background: Prospective trial registration is a powerful tool to prevent reporting bias. We aimed to determine the extent to which published randomized controlled trials (RCTs) were registered and registered prospectively.
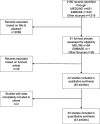
Methods: We searched MEDLINE and EMBASE from January 2005 to October 2017; we also screened all articles cited by or citing included and excluded studies, and the reference lists of related reviews. We included studies that examined published RCTs and evaluated their registration status, regardless of medical specialty or language. We excluded studies that assessed RCT registration status only through mention of registration in the published RCT, without searching registries or contacting the trial investigators. Two independent reviewers blinded to the other's work performed the selection. Following PRISMA guidelines, two investigators independently extracted data, with discrepancies resolved by consensus. We calculated pooled proportions and 95% confidence intervals using random-effects models.
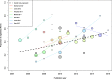
Results: We analyzed 40 studies examining 8773 RCTs across a wide range of clinical specialties. The pooled proportion of registered RCTs was 53% (95% confidence interval 44% to 58%), with considerable between-study heterogeneity. A subset of 24 studies reported data on prospective registration across 5529 RCTs. The pooled proportion of prospectively registered RCTs was 20% (95% confidence interval 15% to 25%). Subgroup analyses showed that registration was higher for industry-supported and larger RCTs. A meta-regression analysis across 19 studies (5144 RCTs) showed that the proportion of registered trials significantly increased over time, with a mean proportion increase of 27%, from 25 to 52%, between 2005 and 2015.
Conclusions: The prevalence of trial registration has increased over time, but only one in five published RCTs is prospectively registered, undermining the validity and integrity of biomedical research.
Keywords: Randomized controlled trials; Registration; Reporting bias.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

