Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer's disease
- PMID: 30322407
- PMCID: PMC6190663
- DOI: 10.1186/s40478-018-0606-1
Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer's disease
Abstract
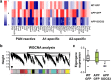
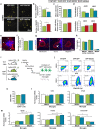
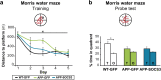
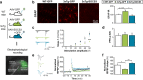
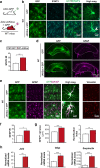
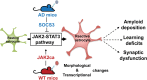
Astrocyte reactivity and neuroinflammation are hallmarks of CNS pathological conditions such as Alzheimer's disease. However, the specific role of reactive astrocytes is still debated. This controversy may stem from the fact that most strategies used to modulate astrocyte reactivity and explore its contribution to disease outcomes have only limited specificity. Moreover, reactive astrocytes are now emerging as heterogeneous cells and all types of astrocyte reactivity may not be controlled efficiently by such strategies.Here, we used cell type-specific approaches in vivo and identified the JAK2-STAT3 pathway, as necessary and sufficient for the induction and maintenance of astrocyte reactivity. Modulation of this cascade by viral gene transfer in mouse astrocytes efficiently controlled several morphological and molecular features of reactivity. Inhibition of this pathway in mouse models of Alzheimer's disease improved three key pathological hallmarks by reducing amyloid deposition, improving spatial learning and restoring synaptic deficits.In conclusion, the JAK2-STAT3 cascade operates as a master regulator of astrocyte reactivity in vivo. Its inhibition offers new therapeutic opportunities for Alzheimer's disease.
Keywords: Alzheimer’s disease; JAK2-STAT3 pathway; Mouse models; Neuroinflammation; Reactive astrocytes; Signaling cascades; Viral vectors.
Conflict of interest statement
Ethics approval and consent to participate
All experimental protocols were reviewed and approved by the local ethics committee (CETEA N°44) and submitted to the French Ministry of Education and Research (Approvals # APAFIS#4565–20 16031711426915 v3, APAFIS#4503–2016031409023019). They were performed in a facility authorized by local authorities (authorization #B92–032-02), in strict accordance with recommendations of the European Union (2010–63/EEC).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

