Omalizumab Treatment for Atopic Severe Persistant Asthma: A Single-Center, Long-Term, Real-Life Experience with 38 Patients
- PMID: 30322442
- PMCID: PMC6196910
- DOI: 10.5152/TurkThoracJ.2018.17109
Omalizumab Treatment for Atopic Severe Persistant Asthma: A Single-Center, Long-Term, Real-Life Experience with 38 Patients
Abstract
Objectives: Omalizumab is a monoclonal antibody that is used as add-on therapy for treating moderate-to-severe persistant atopic asthma in patients with persistant symptoms and frequent exacerbations, despite step 4 treatment according to GINA guidelines. Real-life studies on omalizumab treatment are limited in Turkey. Thus, the present study aims to assess the clinical efficacy and treatment outcomes of omalizumab in patients with atopic severe persistant asthma.
Materials and methods: Patients with atopic severe persistant asthma who were treated with omalizumab between 2009 and 2017 were retrospectively evaluated. Baseline and last results of the following variables were compared: symptom scores (GINA categorical), controller medications, blood eosinophil counts, forced expiratory volume in 1 second (FEV1) values, and the number of exacerbations that were treated with systemic corticosteroids for at least 3 days within the last 1 year. The effect of coexisting aspirin-exacerbated respiratory disease (AERD) on these parameters was also analyzed. Step-down of other asthma medications was attempted in patients with symptom control and in those without an exacerbation history within the last 6 months.
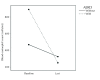
Results: Thirty-eight patients (mean age, 50 years; females, 30) were included in this study, of whom four showed AERD. After treating with a mean time of 30±22.1 (min: 6, max: 92) months, 26 (68%) patients showed complete controlled disease and 12 (32%) showed partly controlled disease, of whom all had uncontrolled disease before. Mean exacerbation rates within the last 1 year decreased by approximately 76% (9.4±8.4 vs. 1.8±1.5; p<0.001) and FEV1 values increased by approximately 14% (2075±729 vs. 2321±800 cc; p=0.001) compared with baseline levels. Although the reduction in eosinophil count was not significant in all patients (503.8±524.8 vs. 370.8±314.5; p=0.134), repeated measures analysis of variance revealed a more prominent reduction in eosinophil count in the AERD group than in the non-AERD group, independent from the treatment period (F: 4.23, p=0.049). The mean inhaled corticosteroid dose (budesonide eq., 1063±397 vs. 958±439 mcg; p=0.084), the number of other controller medications, and the number of patients with long-term systemic steroid use decreased after omalizumab treatment. No serious adverse events were recorded during the follow-up period.
Conclusion: Our results confirm that omalizumab significantly improves disease control and is a safe add-on therapy. In addition, in suitable patients with controlled disease over time, the step-down of other asthma medications will be appropriate.
Conflict of interest statement
Figures
References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2017. [Accessed November 25, 2017]. Available from: www.ginasthma.org.
LinkOut - more resources
Full Text Sources
