The gut microbiome: Relationships with disease and opportunities for therapy
- PMID: 30322864
- PMCID: PMC6314516
- DOI: 10.1084/jem.20180448
The gut microbiome: Relationships with disease and opportunities for therapy
Abstract
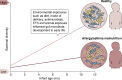
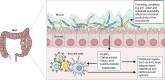
Over the past decade, our view of human-associated microbes has expanded beyond that of a few species toward an appreciation of the diverse and niche-specialized microbial communities that develop in the human host with chronological age. The largest reservoir of microbes exists in the distal gastrointestinal tract, both in the lumen, where microbes facilitate primary and secondary metabolism, and on mucosal surfaces, where they interact with host immune cell populations. While local microbial-driven immunomodulation in the gut is well described, more recent studies have demonstrated a role for the gut microbiome in influencing remote organs and mucosal and hematopoietic immune function. Unsurprisingly, therefore, perturbation to the composition and function of the gut microbiota has been associated with chronic diseases ranging from gastrointestinal inflammatory and metabolic conditions to neurological, cardiovascular, and respiratory illnesses. Considerable effort is currently focused on understanding the natural history of microbiome development in humans in the context of health outcomes, in parallel with improving our knowledge of microbiome-host molecular interactions. These efforts ultimately aim to develop effective approaches to rehabilitate perturbed human microbial ecosystems as a means to restore health or prevent disease. This review details the role of the gut microbiome in modulating host health with a focus on immunomodulation and discusses strategies for manipulating the gut microbiome for the management or prevention of chronic inflammatory conditions.
© 2018 Durack and Lynch.
Figures


References
-
- Ahmadizar F., Vijverberg S.J.H., Arets H.G.M., de Boer A., Turner S., Devereux G., Arabkhazaeli A., Soares P., Mukhopadhyay S., Garssen J., et al. 2017. Early life antibiotic use and the risk of asthma and asthma exacerbations in children. Pediatr. Allergy Immunol. 28:430–437. 10.1111/pai.12725 - DOI - PubMed
-
- Alard J., Lehrter V., Rhimi M., Mangin I., Peucelle V., Abraham A.L., Mariadassou M., Maguin E., Waligora-Dupriet A.J., Pot B., et al. 2016. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ. Microbiol. 18:1484–1497. 10.1111/1462-2920.13181 - DOI - PubMed
-
- Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., Giammaria P., Reali L., Anania F., and Nobili V.. 2014. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 39:1276–1285. 10.1111/apt.12758 - DOI - PMC - PubMed
-
- Allais L., Kerckhof F.M., Verschuere S., Bracke K.R., De Smet R., Laukens D., Van den Abbeele P., De Vos M., Boon N., Brusselle G.G., et al. 2016. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 18:1352–1363. 10.1111/1462-2920.12934 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

