The role of the gustatory cortex in incidental experience-evoked enhancement of later taste learning
- PMID: 30322892
- PMCID: PMC6191014
- DOI: 10.1101/lm.048181.118
The role of the gustatory cortex in incidental experience-evoked enhancement of later taste learning
Abstract
The strength of learned associations between pairs of stimuli is affected by multiple factors, the most extensively studied of which is prior experience with the stimuli themselves. In contrast, little data is available regarding how experience with "incidental" stimuli (independent of any conditioning situation) impacts later learning. This lack of research is striking given the importance of incidental experience to survival. We have recently begun to fill this void using conditioned taste aversion (CTA), wherein an animal learns to avoid a taste that has been associated with malaise. We previously demonstrated that incidental exposure to salty and sour tastes (taste preexposure-TPE) enhances aversions learned later to sucrose. Here, we investigate the neurobiology underlying this phenomenon. First, we use immediate early gene (c-Fos) expression to identify gustatory cortex (GC) as a site at which TPE specifically increases the neural activation caused by taste-malaise pairing (i.e., TPE did not change c-Fos induced by either stimulus in isolation). Next, we use site-specific infection with the optical silencer Archaerhodopsin-T to show that GC inactivation during TPE inhibits the expected enhancements of both learning and CTA-related c-Fos expression, a full day later. Thus, we conclude that GC is almost certainly a vital part of the circuit that integrates incidental experience into later associative learning.
© 2018 Flores et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
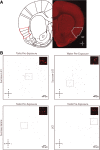

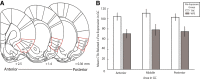
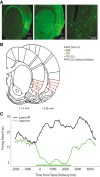

References
-
- Ahlers RH, Best PJ. 1971. Novelty vs temporal contiguity in learned taste aversions. Psychon Sci 25: 34–36.
-
- Allen GV, Saper CB, Hurley KM, Cechetto DF. 1991. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311: 1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
