Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis
- PMID: 30322946
- PMCID: PMC6217390
- DOI: 10.1073/pnas.1811974115
Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis
Abstract
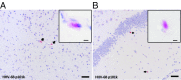
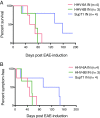
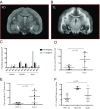
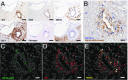
Pathogens, particularly human herpesviruses (HHVs), are implicated as triggers of disease onset/progression in multiple sclerosis (MS) and other neuroinflammatory disorders. However, the time between viral acquisition in childhood and disease onset in adulthood complicates the study of this association. Using nonhuman primates, we demonstrate that intranasal inoculations with HHV-6A and HHV-6B accelerate an MS-like neuroinflammatory disease, experimental autoimmune encephalomyelitis (EAE). Although animals inoculated intranasally with HHV-6 (virus/EAE marmosets) were asymptomatic, they exhibited significantly accelerated clinical EAE compared with control animals. Expansion of a proinflammatory CD8 subset correlated with post-EAE survival in virus/EAE marmosets, suggesting that a peripheral (viral?) antigen-driven expansion may have occurred post-EAE induction. HHV-6 viral antigen in virus/EAE marmosets was markedly elevated and concentrated in brain lesions, similar to previously reported localizations of HHV-6 in MS brain lesions. Collectively, we demonstrate that asymptomatic intranasal viral acquisition accelerates subsequent neuroinflammation in a nonhuman primate model of MS.
Keywords: EAE; human herpesvirus 6; marmoset; multiple sclerosis; viral trigger.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Reply to Zahednasab et al.: HHV-6 and marmoset EAE.Proc Natl Acad Sci U S A. 2018 Dec 26;115(52):E12127. doi: 10.1073/pnas.1818755115. Epub 2018 Dec 11. Proc Natl Acad Sci U S A. 2018. PMID: 30538192 Free PMC article. No abstract available.
-
Role of HHV-6 subtypes in accelerating EAE progression.Proc Natl Acad Sci U S A. 2018 Dec 26;115(52):E12126. doi: 10.1073/pnas.1817967115. Epub 2018 Dec 11. Proc Natl Acad Sci U S A. 2018. PMID: 30538193 Free PMC article. No abstract available.
References
-
- Compston A, Lassman H, McDonald I. McAlpine’s Multiple Sclerosis, 4th Ed Churchill Livingstone/Elsevier; London: 2006. The story of multiple sclerosis.
-
- Merelli E, et al. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J Neurol. 1997;244:450–454. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

