Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: key role of ATM in the differential radiation response
- PMID: 30323167
- PMCID: PMC6189042
- DOI: 10.1038/s41419-018-1095-7
Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: key role of ATM in the differential radiation response
Abstract
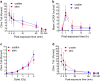
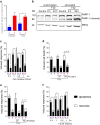
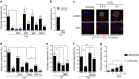
Activation of T cells, a major fraction of peripheral blood lymphocytes (PBLCS), is essential for the immune response. Genotoxic stress resulting from ionizing radiation (IR) and chemical agents, including anticancer drugs, has serious impact on T cells and, therefore, on the immune status. Here we compared the sensitivity of non-stimulated (non-proliferating) vs. CD3/CD28-stimulated (proliferating) PBLC to IR. PBLCs were highly sensitive to IR and, surprisingly, stimulation to proliferation resulted in resistance to IR. Radioprotection following CD3/CD28 activation was observed in different T-cell subsets, whereas stimulated CD34+ progenitor cells did not become resistant to IR. Following stimulation, PBLCs showed no significant differences in the repair of IR-induced DNA damage compared with unstimulated cells. Interestingly, ATM is expressed at high level in resting PBLCs and CD3/CD28 stimulation leads to transcriptional downregulation and reduced ATM phosphorylation following IR, indicating ATM to be key regulator of the high radiosensitivity of resting PBLCs. In line with this, pharmacological inhibition of ATM caused radioresistance of unstimulated, but not stimulated, PBLCs. Radioprotection was also achieved by inhibition of MRE11 and CHK1/CHK2, supporting the notion that downregulation of the MRN-ATM-CHK pathway following CD3/CD28 activation results in radioprotection of proliferating PBLCs. Interestingly, the crosslinking anticancer drug mafosfamide induced, like IR, more death in unstimulated than in stimulated PBLCs. In contrast, the bacterial toxin CDT, damaging DNA through inherent DNase activity, and the DNA methylating anticancer drug temozolomide induced more death in CD3/CD28-stimulated than in unstimulated PBLCs. Thus, the sensitivity of stimulated vs. non-stimulated lymphocytes to genotoxins strongly depends on the kind of DNA damage induced. This is the first study in which the killing response of non-proliferating vs. proliferating T cells was comparatively determined. The data provide insights on how immunotherapeutic strategies resting on T-cell activation can be impacted by differential cytotoxic effects resulting from radiation and chemotherapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

