A protein functionalization platform based on selective reactions at methionine residues
- PMID: 30323287
- PMCID: PMC6203954
- DOI: 10.1038/s41586-018-0608-y
A protein functionalization platform based on selective reactions at methionine residues
Abstract
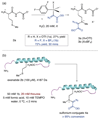
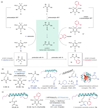
Nature has a remarkable ability to carry out site-selective post-translational modification of proteins, therefore enabling a marked increase in their functional diversity1. Inspired by this, chemical tools have been developed for the synthetic manipulation of protein structure and function, and have become essential to the continued advancement of chemical biology, molecular biology and medicine. However, the number of chemical transformations that are suitable for effective protein functionalization is limited, because the stringent demands inherent to biological systems preclude the applicability of many potential processes2. These chemical transformations often need to be selective at a single site on a protein, proceed with very fast reaction rates, operate under biologically ambient conditions and should provide homogeneous products with near-perfect conversion2-7. Although many bioconjugation methods exist at cysteine, lysine and tyrosine, a method targeting a less-explored amino acid would considerably expand the protein functionalization toolbox. Here we report the development of a multifaceted approach to protein functionalization based on chemoselective labelling at methionine residues. By exploiting the electrophilic reactivity of a bespoke hypervalent iodine reagent, the S-Me group in the side chain of methionine can be targeted. The bioconjugation reaction is fast, selective, operates at low-micromolar concentrations and is complementary to existing bioconjugation strategies. Moreover, it produces a protein conjugate that is itself a high-energy intermediate with reactive properties and can serve as a platform for the development of secondary, visible-light-mediated bioorthogonal protein functionalization processes. The merger of these approaches provides a versatile platform for the development of distinct transformations that deliver information-rich protein conjugates directly from the native biomacromolecules.
Conflict of interest statement
The authors declare no conflict of interest
Figures


Comment in
-
A methionine modification method.Nat Methods. 2018 Dec;15(12):1002. doi: 10.1038/s41592-018-0252-3. Nat Methods. 2018. PMID: 30504878 No abstract available.
References
-
- Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed. 2005;44:7342–7372. - PubMed
-
- Spicer CD, Davis BG. Selective chemical protein modification. Nature Communications. 2014;5:4740. - PubMed
-
- Koniev O, Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem Soc Rev. 2015;44:5495–5551. - PubMed
-
- Dawson PE, Kent SBH. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69:923–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

