A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease
- PMID: 30323548
- PMCID: PMC6174300
- DOI: 10.2147/OPTH.S175065
A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease
Erratum in
-
Erratum: A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease [Corrigendum].Clin Ophthalmol. 2018 Dec 14;12:2637. doi: 10.2147/OPTH.S196843. eCollection 2018. Clin Ophthalmol. 2018. PMID: 30587910 Free PMC article.
-
Erratum: A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease [Corrigendum].Clin Ophthalmol. 2019 Jan 24;13:215. doi: 10.2147/OPTH.S201658. eCollection 2019. Clin Ophthalmol. 2019. PMID: 30774301 Free PMC article.
Abstract
Purpose: The aim of this study was to evaluate the safety and efficacy of OTX-101, a clear nanomicellar aqueous solution of cyclosporine, in the treatment of dry eye disease (DED).
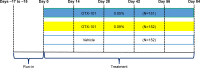
Patients and methods: This was a 12-week multicenter, randomized, prospective, double-masked, vehicle-controlled, dose-ranging clinical trial. Subjects were adults aged ≥18 years, with a total conjunctival staining score of ≥3 and ≤9, and global DED symptom score ≥40 (0-100 visual analogue scale). Following a 14-day vehicle run-in, subjects were randomized in a 1:1:1 ratio to twice daily treatment with OTX-101 0.09%, OTX-101 0.05%, or vehicle for 84 days. Co-primary efficacy end points were changes, from baseline to Day 84, in the total lissamine green conjunctival staining score in the designated study eye and in the global symptom score (both eyes). Secondary end points included total corneal fluorescein staining score, tear breakup time, and Schirmer's test score.
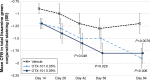
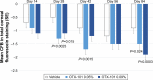
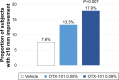
Results: In total, 455 subjects were randomized. Subjects treated with active drug experienced greater improvement in conjunctival staining than vehicle-treated patients (P<0.01 for both concentrations). All groups demonstrated improvements in global symptom score, but there were no differences among groups. Nominally significant differences were found between the active drug arms and vehicle for corneal staining scores and Schirmer's test scores. Most treatment-emergent adverse events were mild in severity; no serious ocular adverse events were reported.
Conclusions: Both concentrations of OTX-101 met the co-primary sign end point (conjunctival staining) but not the co-primary symptom end point. OTX-101 0.09% demonstrated a notable impact on multiple signs of DED relative to vehicle and was well-tolerated.
Keywords: Schirmer’s test; cyclosporine; dry eye disease; inflammation.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. - PubMed
-
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276–283. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

