All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy
- PMID: 30323927
- PMCID: PMC6177463
- DOI: 10.1038/s41377-018-0070-5
All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy
Abstract
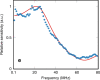
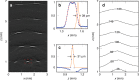
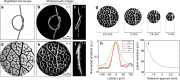
A miniature forward-viewing endoscopic probe that provides high-resolution 3D photoacoustic images is demonstrated. The probe is of outer diameter 3.2 mm and comprised of a transparent Fabry-Pérot (FP) polymer-film ultrasound sensor that is located at the distal end of a rigid optical fiber bundle. Excitation laser pulses are coupled simultaneously into all cores of the bundle and are transmitted through the FP sensor to provide wide-field tissue illumination at the distal end. The resulting photoacoustic waves are mapped in 2D by sequentially scanning the input end of the bundle with an interrogation laser beam in order to individually address different points on the FP sensor. In this way, the sensor acts as a high-density ultrasound array that is comprised of 50,000 individual elements, each of which is 12 µm in diameter, within the 3.2 mm diameter footprint of the probe. The fine spatial sampling that this affords, along with the wide bandwidth (f -3dB = 34 MHz) of the sensor, enables a high-resolution photoacoustic image to be reconstructed. The measured on-axis lateral resolution of the probe was depth-dependent and ranged from 45-170 µm for depths between 1 and 7 mm, and the vertical resolution was 31 µm over the same depth range. The system was evaluated by acquiring 3D images of absorbing phantoms and the microvascular anatomies of a duck embryo and mouse skin. Excellent image fidelity was demonstrated. It is anticipated that this type of probe could find application as a tool for guiding laparoscopic procedures, fetal surgery and other minimally invasive interventions that require a millimeter-scale forward-viewing 3D photoacoustic imaging probe.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Oraevsky Alexander, Karabutov Alexander. Biomedical Photonics Handbook. 2003. Optoacoustic Tomography.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

