Targeting PDK4 inhibits breast cancer metabolism
- PMID: 30323966
- PMCID: PMC6176187
Targeting PDK4 inhibits breast cancer metabolism
Abstract
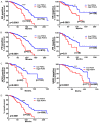
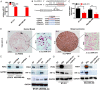
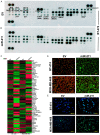
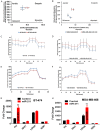
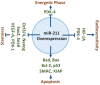
Dysregulated metabolism in the form of aerobic glycolysis occurs in many cancers including breast carcinoma. Here, we report PDK4 (pyruvate dehydrogenase kinase 4) as key enzyme implicated in the control of glucose metabolism and mitochondrial respiration is relatively highly expressed in breast cancers, and its expression correlates with poor patient outcomes. Silencing of PDK4 and ectopic expression of miR-211 attenuates PDK4 expression in breast cancer cells. Interestingly, low miR-211 expression is significantly associated with shorter overall survival and reveals an inverse correlation between expression of miR-211 and PDK4. We have found that depletion of PDK4 by miR-211 shows an oxidative phosphorylation-dominant phenotype consisting of the reduction of glucose with increased expression of PDH and key enzymes of the TCA cycle. miR-211 expression causes alteration of mitochondrial membrane potential and induces mitochondrial apoptosis as observed via IPAD assay. Further, by inhibiting PDK4 expression, miR-211 promotes a phenotype shift towards a pro-glycolytic state evidenced by decreased extracellular acidification rate (ECAR); increased oxygen consumption rate (OCR); and increased spare respiratory capacity in breast cancer cell lines. Taken together this data establishes a molecular connection between PDK4 and miR-211 and suggests that targeting miR-211 to inhibit PDK4 could represent a novel therapeutic strategy in breast cancers.
Keywords: PDK4; Warburg effect; altered metabolism; apoptosis; miR-211.
Conflict of interest statement
None.
Figures

References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. - PMC - PubMed
-
- Li L, Kang L, Zhao W, Feng Y, Liu W, Wang T, Mai H, Huang J, Chen S, Liang Y, Han J, Xu X, Ye Q. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated warburg effect. Cancer Lett. 2017;400:89–98. - PubMed
-
- Velpula KK, Bhasin A, Asuthkar S, Tsung AJ. Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the warburg effect. Cancer Res. 2013;73:7277–89. - PubMed
LinkOut - more resources
Full Text Sources
