Staphylococcus aureus alpha toxin activates Notch in vascular cells
- PMID: 30324336
- PMCID: PMC6360126
- DOI: 10.1007/s10456-018-9650-5
Staphylococcus aureus alpha toxin activates Notch in vascular cells
Abstract
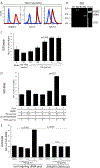
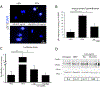
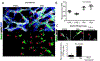
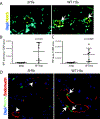
Staphylococcus aureus infection is one of the leading causes of morbidity in hospitalized patients in the United States, an effect compounded by increasing antibiotic resistance. The secreted agent hemolysin alpha toxin (Hla) requires the receptor A Disintegrin And Metalloproteinase domain-containing protein 10 (ADAM10) to mediate its toxic effects. We hypothesized that these effects are in part regulated by Notch signaling, for which ADAM10 activation is essential. Notch proteins function in developmental and pathological angiogenesis via the modulation of key pathways in endothelial and perivascular cells. Thus, we hypothesized that Hla would activate Notch in vascular cells. Human umbilical vein endothelial cells were treated with recombinant Hla (rHla), Hla-H35L (genetically inactivated Hla), or Hank's solution (HBSS), and probed by different methods. Luciferase assays showed that Hla (0.01 µg/mL) increased Notch activation by 1.75 ± 0.5-fold as compared to HBSS controls (p < 0.05), whereas Hla-H35L had no effect. Immunocytochemistry and Western blotting confirmed these findings and revealed that ADAM10 and γ-secretase are required for Notch activation after inhibitor and siRNA assays. Retinal EC in mice engineered to express yellow fluorescent protein (YFP) upon Notch activation demonstrated significantly greater YFP intensity after Hla injection than controls. Aortic rings from Notch reporter mice embedded in matrix and incubated with rHla or Hla-H35L demonstrate increased Notch activation occurs at tip cells during sprouting. These mice also had higher skin YFP intensity and area of expression after subcutaneous inoculation of S. aureus expressing Hla than a strain lacking Hla in both EC and pericytes assessed by microscopy. Human liver displayed strikingly higher Notch expression in EC and pericytes during S. aureus infection by immunohistochemistry than tissues from uninfected patients. In sum, our results demonstrate that the S. aureus toxin Hla can potently activate Notch in vascular cells, an effect which may contribute to the pathobiology of infection with this microorganism.
Keywords: Alpha-toxin; HUVEC; Notch; Staphylococcus aureus.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

