Differential cerebral hemometabolic responses to blood transfusions in adults and children with sickle cell anemia
- PMID: 30324698
- PMCID: PMC6330124
- DOI: 10.1002/jmri.26213
Differential cerebral hemometabolic responses to blood transfusions in adults and children with sickle cell anemia
Abstract
Background: Blood transfusions are administered to children and adults with sickle cell anemia (SCA) for secondary stroke prevention, or as treatment for recurrent pain crises or acute anemia, but transfusion effects on cerebral hemodynamics and metabolism are not well-characterized.
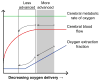
Purpose: To compare blood transfusion-induced changes in hemometabolic parameters, including oxygen extraction fraction (OEF) and cerebral blood flow (CBF), within and between adults and children with SCA.
Study type: Prospective, longitudinal study.
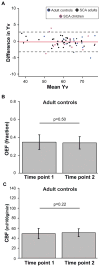
Subjects: Adults with SCA (n = 16) receiving simple (n = 7) or exchange (n = 9) transfusions and children with SCA (n = 11) receiving exchange transfusions were scanned once when hematocrit was near nadir and again within 7 days of transfusion. Adult controls without SCA or sickle trait (n = 7) were scanned twice on separate days.
Field strength/sequence: 3.0T T1 -weighted, T2 -weighted, and T2 -relaxation-under-spin-tagging (TRUST) imaging, and phase contrast angiography.
Assessment: Global OEF was computed as the relative difference between venous oxygenation (from TRUST) and arterial oxygenation (from pulse oximetry). Global CBF was computed as total blood flow to the brain normalized by intracranial tissue volume.
Statistical tests: Hemometabolic variables were compared using two-sided Wilcoxon signed-rank tests; associations were analyzed using two-sided Spearman's correlation testing.
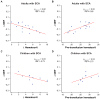
Results: In adults with SCA, posttransfusion OEF = 0.38 ± 0.05 was lower (P = 0.001) than pretransfusion OEF = 0.45 ± 0.09. A change in OEF was correlated with increases in hematocrit (P = 0.02; rho = -0.62) and with pretransfusion hematocrit (P = 0.02; rho = 0.65). OEF changes after transfusion were greater (P = 0.002) in adults receiving simple versus exchange transfusions. Posttransfusion CBF = 77.7 ± 26.4 ml/100g/min was not different (P = 0.27) from pretransfusion CBF = 82.3 ± 30.2 ml/100g/min. In children with SCA, both posttransfusion OEF = 0.28 ± 0.04 and CBF = 76.4 ± 26.4 were lower than pretransfusion OEF = 0.36 ± 0.06 (P = 0.004) and CBF = 96.4 ± 16.5 (P = 0.004).
Data conclusion: Cerebral OEF reduces following transfusions in adults and children with SCA. CBF reduces following transfusions more often in children compared to adults, indicating that vascular reserve capacity may remain near exhaustion posttransfusion in many adults.
Level of evidence: 2 Technical Efficacy Stage 5 J. Magn. Reson. Imaging 2019;49:466-477.
Keywords: blood transfusion; cerebral blood flow; oxygen extraction fraction; phase contrast angiography; sickle cell anemia.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

