Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis
- PMID: 30325017
- PMCID: PMC6517135
- DOI: 10.1002/14651858.CD007419.pub6
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis
Update in
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. Cochrane Database Syst Rev. 2023. PMID: 38275741 Free PMC article.
Abstract
Background: Diabetic macular oedema (DMO) is a common complication of diabetic retinopathy. Antiangiogenic therapy with anti-vascular endothelial growth factor (anti-VEGF) can reduce oedema, improve vision and prevent further visual loss. These drugs have replaced laser photocoagulation as the standard of care for people with DMO.
Objectives: The 2014 update of this review found high-quality evidence of benefit with anti-VEGF modalities, compared to laser photocoagulation, for the treatment of DMO. The objective of this updated review is to compare the effectiveness and safety of the different anti-VEGF drugs using network meta-analysis methods.
Search methods: We searched various electronic databases on 26 April 2017.
Selection criteria: We included randomised controlled trials (RCTs) that compared any anti-angiogenic drug with an anti-VEGF mechanism of action versus another anti-VEGF drug, another treatment, sham or no treatment in people with DMO.
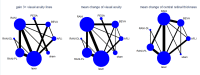
Data collection and analysis: We used standard Cochrane methods for pair-wise meta-analysis and we augmented this evidence using network meta-analysis methods. We focused on the relative efficacy and safety of the three most commonly used drugs as interventions of direct interest for practice: aflibercept and ranibizumab, used on-label; and off-label bevacizumab.We collected data on three efficacy outcomes (gain of 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters; mean change in best-corrected visual acuity (BCVA); mean change in central retinal thickness (CRT)), three safety outcomes (all severe systemic adverse events (SSAEs); all-cause death; arterial thromboembolic events) and quality of life.We used Stata 'network' meta-analysis package for all analyses. We investigated the risk of bias of mixed comparisons based on the variance contribution of each study, having assigned an overall risk of bias to each study.
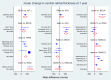
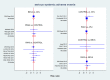
Main results: Twenty-four studies included 6007 participants with DMO and moderate vision loss, of which two studies randomised 265 eyes of 230 participants and one was a cross-over study on 56 participants (62 eyes) that was treated as a parallel-arm trial. Data were collected on drugs of direct interest from three studies on aflibercept (975 eyes), eight studies on bevacizumab (515 eyes), and 14 studies on ranibizumab (1518 eyes). As treatments of indirect interest or legacy treatment we included three studies on pegaptanib (541 eyes), five studies on ranibizumab plus prompt laser (557 eyes), one study on ranibizumab plus deferred laser (188 eyes), 13 studies on laser photocoagulation (936 eyes) and six studies on sham treatment (793 eyes).Aflibercept, bevacizumab and ranibizumab were all more effective than laser for improving vision by 3 or more lines after one year (high-certainty evidence). Approximately one in 10 people improve vision with laser, and about three in 10 people improve with anti-VEGF treatment: risk ratio (RR) versus laser 3.66 (95% confidence interval (CI) 2.79 to 4.79) for aflibercept; RR 2.47 (95% CI 1.81 to 3.37) for bevacizumab; RR 2.76 (95% CI 2.12 to 3.59) for ranibizumab. On average there was no change in visual acuity (VA) with laser after one year, compared with a gain of 1 or 2 lines with anti-VEGF treatment: laser versus aflibercept mean difference (MD) -0.20 (95% CI -0.22 to -0.17) logMAR; versus bevacizumab MD -0.12 (95% CI -0.15 to -0.09) logMAR; versus ranibizumab MD -0.12 (95% CI -0.14 to -0.10) logMAR. The certainty of the evidence was high for the comparison of aflibercept and ranibizumab with laser and moderate for bevacizumab comparison with laser due to inconsistency between the indirect and direct evidence.People receiving ranibizumab were less likely to gain 3 or more lines of VA at one year compared with aflibercept: RR 0.75 (95% CI 0.60 to 0.94), moderate-certainty evidence. For every 1000 people treated with aflibercept, 92 fewer would gain 3 or more lines of VA at one year if treated with ranibizumab (22 to 148 fewer). On average people receiving ranibizumab had worse VA at one year (MD 0.08 logMAR units, 95% CI 0.05 to 0.11), moderate-certainty evidence; and higher CRT (MD 39 µm, 95% CI 2 µm to 76 µm; low-certainty evidence). Ranibizumab and bevacizumab were comparable with respect to aflibercept and did not differ in terms of VA: RR of gain of 3 or more lines of VA at one year 1.11 (95% CI 0.87 to 1.43), moderate-certainty evidence, and difference in change in VA was 0.00 (95% CI -0.02 to 0.03) logMAR, moderate-certainty evidence. CRT reduction favoured ranibizumab by -29 µm (95% CI -58 µm to -1 µm, low-certainty evidence). There was no evidence of overall statistical inconsistency in our analyses.The previous version of this review found moderate-certainty evidence of good safety of antiangiogenic drugs versus control. This update used data at the longest available follow-up (one or two years) and found that aflibercept, ranibizumab and bevacizumab do not differ regarding systemic serious adverse events (SSAEs) (moderate- or high-certainty evidence). However, risk of bias was variable, loop inconsistency could be found and estimates were not precise enough on relative safety regarding less frequent events such as arterial thromboembolic events or death (low- or very low-certainty evidence).Two-year data were available and reported in only four RCTs in this review. Most industry-sponsored studies were open-label after one year. One large publicly-funded study compared the three drugs at two years and found no difference.
Authors' conclusions: Anti-VEGF drugs are effective at improving vision in people with DMO with three to four in every 10 people likely to experience an improvement of 3 or more lines VA at one year. Aflibercept may confer some advantage over ranibizumab and bevacizumab in people with DMO at one year in visual and anatomic terms but it is unclear whether this applies to the long-term. There is a need for more evidence on the long-term (greater than two years) comparative effects of these anti-VEGF agents. Evidence from RCTs may not apply to real-world practice, where people in need of antiangiogenic treatment are often under-treated and under-monitored.We found no signals of differences in overall safety between the three antiangiogenic drugs that are currently available to treat DMO, but our estimates are imprecise for cardiovascular events and death.
Conflict of interest statement
Gianni Virgili: none known Mariacristina Parravano received payment for participating on the Advisory Board for Allergan, Bayer and Novartis. Jennifer Evans: none known Iris Gordon: none known Ersilia Lucenteforte: none known
Figures








Update of
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD007419. doi: 10.1002/14651858.CD007419.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. PMID: 28639415 Free PMC article. Updated.
References
References to studies included in this review
Ahmadieh 2008 {published data only}
-
- Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo‐controlled, randomized clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(4):483‐9. - PubMed
Azad 2012 {published data only}
BOLT 2010 {published data only}
-
- Michaelides M, Kaines A, Hamilton RD, Fraser‐Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12‐month data: report 2. Ophthalmology 2010;117(6):1078‐86. - PubMed
-
- Rajendram R, Fraser‐Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2‐year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24‐month data: report 3. Archives of Ophthalmology 2012;130(8):972‐9. - PubMed
DA VINCI 2011 {published data only}
-
- Do DV, Nguyen QD, Boyer D, Schmidt‐Erfurth U, Brown DM, Vitti R, et al. One‐year outcomes of the DA VINCI Study of VEGF Trap‐Eye in eyes with diabetic macular edema. Ophthalmology 2012;119(8):1658‐65. - PubMed
-
- Do DV, Schmidt‐Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, et al. The DAVINCI Study: phase 2 primary results of VEGF Trap‐Eye in patients with diabetic macular edema. Ophthalmology 2011;118(9):1819‐26. - PubMed
DRCRnet 2010 {published data only}
-
- Anonymous. Erratum: Patient‐reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: Randomized clinical trial (JAMA Ophthalmology (2013) 131:10 (1339‐1347) DOI: 10.1001/jamaophthalmol.2013.4592). JAMA Ophthalmology 2013;131(12):1652. - PubMed
DRCRnet 2015 {published data only}
Ekinci 2014 {published data only}
-
- Ekinci M, Ceylan E, Cakici O, Tanyildiz B, Olcaysu O, Cagatay HH. Treatment of macular edema in diabetic retinopathy: Comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Review of Ophthalmology 2014;9(2):139‐43.
Ishibashi 2014 {published data only}
-
- Ishibashi T, Yuzawa M, Yoshimura N, Ohji M, Ishida S, Isogawa N, et al. Japan phase 3 study of pegaptanib sodium in patients with diabetic macular edema. Nippon Ganka Gakkai Zasshi 2014;118(9):773‐82. - PubMed
-
- NCT01100307. A phase 3 study to compare the efficacy and safety of 0.3 mg pegaptanib sodium to sham Injections In subjects with diabetic macular edema [A phase 3, randomized, controlled, double‐masked, multi‐center, comparative, in parallel roups (for 24 weeks), to compare the efficacy and safety of 0.3 mg pegaptanib sodium, with sham injections, and open study (for 30 weeks) to confirm the safety of 0.3 mg pegaptanib sodium in subjects with diabetic macular edema (DME)]. clinicaltrials.gov/show/NCT01100307 (first received 7 April 2010).
Korobelnik 2014 {published data only}
-
- Korobelnik JF, Do DV, Schmidt‐Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121(11):2247‐54. - PubMed
Lopez‐Galvez 2014 {published and unpublished data}
-
- Lopez‐Galvez MI, Arias L, Roura M. Efficacy and safety profile of ranibizumab versus laser photocoagulation in patients with diabetic macular edema. Re‐Des Study. Ophthalmologica 2014;232:115.
LUCIDATE 2014 {published data only}
-
- Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). American Journal of Ophthalmology 2014; Vol. 157, issue 5:960‐70. - PubMed
Macugen 2005 {published data only}
-
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747‐57. - PubMed
Macugen 2011 {published data only}
-
- Loftus JV, Sultan MB, Pleil AM, Macugen 1013 Study Group. Changes in vision and health‐related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Investigative Ophthalmology and Visual Science 2011;52(10):7498‐505. - PubMed
-
- Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS, Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double‐masked, 2‐year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011;118(6):1107‐18. - PubMed
Nepomuceno 2013 {published data only}
-
- Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, et al. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. American Journal of Ophthalmology 2013;156(3):502‐10. - PubMed
READ2 2009 {published data only}
-
- Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, et al. Ranibizumab for edema of the macula in diabetes study: 3‐year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmology 2013;131(2):139‐45. - PubMed
-
- Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 2009;116(11):2175‐81. - PubMed
-
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two‐year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 2010;117(11):2146‐51. - PubMed
RELATION 2012 {unpublished data only}
-
- CRFB002DD13. A 12‐month, two‐armed, randomized, double‐masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
-
- NCT01131585. A 12‐month, two‐armed, randomized, double‐masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. clinicaltrials.gov/show/NCT01131585 (first received 25 May 2010).
RESOLVE 2010 {published data only}
-
- CRFB002D2201. A randomized, double‐masked, multicenter, phase II study assessing the safety and efficacy of two concentrations of ranibizumab (intravitreal injections) compared with non‐treatment control for the treatment of diabetic macular edema with center involvement. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
RESPOND 2013 {unpublished data only}
-
- Berger A, Sheidow T, Li R, Rehel B, Takacsy F, Courseau AS. A Canadian 12‐month, phase IIIb study of ranibizumab combination or monotherapy in visual impairment due to diabetic macular edema: Preliminary analysis ("RESPOND"). 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings; 2013 Oct 17‐19; Montreal. 2013.
-
- CRFB002DCA05. A Canadian 12‐month, prospective, randomized, open‐label, multicenter, phase IIIb study assessing the efficacy, safety and cost of ranibizumab as combination and monotherapy in patients with visual impairment due to diabetic macular edema. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
RESTORE 2011 {published data only}
-
- Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, et al. Two‐year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 2013; Vol. 120, issue 10:2004‐12. - PubMed
-
- Mitchell P, Bandello F, Schmidt‐Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615–62. - PubMed
-
- Mitchell P, Bressler N, Tolley K, Gallagher M, Petrillo J, Ferreira A, et al. Patient‐reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmology 2013;131(10):1339‐47. - PubMed
REVEAL 2015 {published data only}
-
- Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema. Ophthalmology 2015;122(7):1402‐15. - PubMed
RISE‐RIDE {published data only}
-
- Bressler NM, Varma R, Suñer IJ, Dolan CM, Ward J, Ehrlich JS, et al. Vision‐related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISE. Ophthalmology 2014;121(12):2461‐72. - PubMed
-
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long‐term outcomes of ranibizumab therapy for diabetic macular edema: the 36‐month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013‐22. - PubMed
-
- Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long‐term effects of ranibizumab on diabetic retinopathy severity and progression. Archives of Ophthalmology 2012;130(9):1145‐52. - PubMed
-
- Mieler WF, Kim JE, Yau L, Ehrlich JS. Earlier treatment is important in diabetic macular edema: Outcomes from phase III trials of intravitreal ranibizumab. 73rd Scientific sessions of the American Diabetes Association; 2013 Jun 21‐25; Chicago. 2013.
-
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789‐801. - PubMed
Soheilian 2007 {published data only}
-
- Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two‐year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina 2012;32(2):314‐21. - PubMed
-
- Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, et al. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina 2007;27(9):1187‐95. - PubMed
-
- Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116(6):1142‐50. - PubMed
Turkoglu 2015 {published data only}
-
- Turkoglu EB, Celık E, Aksoy N, Bursalı O, Ucak T, Alagoz G. Changes in vision related quality of life in patients with diabetic macular edema: ranibizumab or laser treatment?. Journal of Diabetes and its Complications 2015;29(4):540‐3. - PubMed
References to studies excluded from this review
Ahmadieh 2013 {published data only}
-
- Ahmadieh H, Nourinia R, Hafezi‐Moghadam A. Intravitreal fasudil combined with bevacizumab for persistent diabetic macular edema. JAMA Ophthalmology 2013;131(7):923‐4. - PubMed
CRFB002DFR08 (LUDIC) {unpublished data only}
-
- CRFB002DFR08. Open‐label, multicenter, study of the efficacy and safety of Lucentis® (ranibizumab 0.5 mg) in diabetic patients presenting a visual impairment due to diabetic macular edema in current medical practice (LUDIC). Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=9803 (accessed 28 May 2014).
CRFB002DGB14 (RELIGHT) {unpublished data only}
-
- CRFB002DGB14. RELIGHT ‐ Ranibizumab treatment of diabetic macular oEdema with bimonthLy monItorinG after a pHase of initial Treatment. A UK, 18‐month, prospective, open‐label, multicentre, single‐arm Phase IIIb study, with 12‐month primary endpoint, assessing the efficacy and safety of Ranibizumab in patients with visual impairment. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/products.jsp?d... (accessed 28 May 2014).
CRFB002DNO02 (PTIMAL) {unpublished data only}
-
- CRFB002DNO02. An open‐label, prospective, multicenter, uncontrolled, Proof of concept study assessing the efficacy of Lucentis (ranibizumab) administered by an individualized “treat and extend” dosing regimen in patients with visual impairment due to dIabetic macular edema. Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=10423 (accessed 28 May 2014).
DRCRnet 2007 {published data only}
DRCRnet 2011 {published data only}
-
- Diabetic Retinopathy Clinical Research Network, Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, et al. Randomized trial evaluating short‐term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011;31(6):1009‐27. - PMC - PubMed
DRCRnet 2012 {published data only}
-
- Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three‐year randomized trial results. Ophthalmology 2012; Vol. 119, issue 11:2312‐8. - PMC - PubMed
Faghihi 2008 {published data only}
-
- Faghihi H, Roohipoor R, Mohammadi SF, Hojat‐Jalali K, Mirshahi A, Lashay A, et al. Intravitreal bevacizumab versus combined bevacizumab‐triamcinolone versus macular laser photocoagulation in diabetic macular edema. European Journal of Ophthalmology 2008;18(6):941‐8. - PubMed
NCT02985619 (BEVATAAC) {published data only}
-
- NCT02985619. Bevacizumabe or triamcinolone for persistent diabetic macular edema (BEVATAAC) [Randomized trial evaluating bevacizumabe or triamcinolone for persistent diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02985619 (first received 1 December 2016).
Paccola 2008 {published data only}
-
- Paccola L, Costa RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). British Journal of Ophthalmology 2008;92(1):76‐80. - PubMed
Solaiman 2010 {published data only}
-
- Solaiman KA, Diab MM, Abo‐Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina 2010;30(10):1638‐45. - PubMed
Zehetner 2013 {published data only}
-
- Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age‐related macular degeneration, and in patients with diabetic macular oedema. British Journal of Ophthalmology 2013;97(4):454‐9. - PubMed
References to studies awaiting assessment
Chen 2016 {published data only}
-
- Chen ZX, Fu JS, Song W, Wang CX, Zhang YL. Effect analysis of ranibizumab with laser photocoagulation therapy for diabetic macular edema. International Eye Science 2016;16(4):706‐8.
Fouda 2017 {published data only}
Huang 2016 {published data only}
-
- Huang JD, Song ZY. Clinical study of grid pattern laser photocoagulation with ranibizumab for diabetic macular edema. International Eye Science 2016;16(3):493‐5.
Jovanovic 2015 {published data only}
-
- Jovanović S, Čanadanović V, Sabo A, Grgić Z, Mitrović M, Rakić D. Intravitreal bevacizumab injection alone or combined with macular photocoagulation compared to macular photocoagulation as primary treatment of diabetic macular edema. Vojnosanitetski Pregled 2015;72(10):876‐82. - PubMed
NCT00387582 {published data only}
-
- NCT00387582. Efficacy study of lucentis in the treatment of diabetic macular edema ‐ a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema [Lucentis in the treatment of macular edema ‐ a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema]. clinicaltrials.gov/show/NCT00387582 (first received 11 October 2006).
NCT00997191 (IBeTA) {published data only}
-
- NCT00997191. Intravitreal bevacizumab and intravitreal triamcinolone associated to laser photocoagulation for diabetic macular edema (IBeTA). clinicaltrials.gov/show/NCT00997191 (first received 15 October 2009).
NCT01445899 (MATISSE) {published data only}
-
- NCT01445899. PF‐04523655 dose escalation study, and evaluation of PF‐04523655 with/without ranibizumab in diabetic macular edema (DME) (MATISSE) [An open‐label dose escalation study of PF‐04523655 (Stratum I) combined with a prospective, randomized, double‐masked, multi‐center, controlled study (Stratum II) evaluating the efficacy and safety of PF‐04523655 alone and in combination with ranibizumab versus ranibizumab alone in diabetic macular edema (MATISSE STUDY)]. clinicaltrials.gov/ct2/show/study/NCT01445899 (first received 30 September 2011).
NCT01565148 (IDEAL) {published data only}
-
- NCT01565148. A randomized, multi‐center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo‐007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema (the iDEAL Study) (iDEAL) [A randomized, multi‐center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo‐007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema with involvement of the foveAL center (the iDEAL Study)]. clinicaltrials.gov/ct2/show/study/NCT01565148 (first received 15 March 2012).
References to ongoing studies
ChiCTR‐TRC‐12002417 {unpublished data only}
-
- ChiCTR‐TRC‐12002417. A randomized controlled trial to compare the efficacy and safety of 1) macular laser vs. 2) repeated intravitreal bevacizumab vs. 3) combined repeated intravitreal bevacizumab with macular laser for diabetic macular edema. www.chictr.org.cn/hvshowproject.aspx?id=3319 (accessed 17 September 2014).
NCT01635790 (BRDME) {published data only}
-
- NCT01635790. Comparing the effectiveness and costs of bevacizumab to ranibizumab in patients with diabetic macular edema (BRDME) (BRDME). clinicaltrials.gov/ct2/show/NCT01635790 (first received 28 June 2012).
NCT02194634 {published data only}
-
- NCT02194634. Safety and efficacy study of conbercept in diabetic macular edema (DME) (Sailing). clinicaltrials.gov/ct2/show/NCT02194634 (first received 14 July 2014).
NCT02259088 {published data only}
-
- NCT02259088. A 12‐month, randomized, efficacy and safety study of 0.5 mg ranibizumab vs laser in Chinese DME patients [A 12‐month, randomized, double‐masked, multicenter, laser‐controlled phase III study assessing the efficacy and safety of 0.5 mg ranibizumab dosed PRN in subjects with visual impairment due to diabetic macular edema in Chinese patients]. clinicaltrials.gov/ct2/show/NCT02259088 (first received 3 October 2014).
NCT02348918 {published data only}
-
- NCT02348918. A phase 2 randomized, controlled, double‐masked, multicenter clinical trial designed to evaluate the safety and exploratory efficacy of Luminate® (ALG‐1001) as compared to Avastin® and focal laser photocoagulation in the treatment of diabetic macular edema. clinicaltrials.gov/ct2/show/NCT02348918 (first received 12 January 2015).
NCT02645734 {published data only}
-
- NCT02645734. The effect of bevacizumab and ziv‐aflibercept in diabetic macular edema [The comparison between the therapeutic effect of intravitreal bevacizumab and ziv‐aflibercept in diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02645734 (first received 2 January 2016).
NCT02699450 {published data only}
-
- NCT02699450. A phase 2 study of RO6867461 in participants with center‐involving diabetic macular edema (CI‐DME) (BOULEVARD). clinicaltrials.gov/ct2/show/NCT02699450 (first received 1 March 2016).
NCT02712008 {published data only}
-
- NCT02712008. Anti‐vasculaR endothelial growth factor plUs anti‐angiopoietin 2 in fixed comBination therapY: evaluation for the treatment of diabetic macular edema [A randomized, double‐masked, active‐controlled, phase 2 study of the efficacy, safety, and tolerability of repeated doses of intravitreal REGN910‐3 in patients with diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02712008 (first received 14 March 2016).
Additional references
Aiello 2005
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. New England Journal of Medicine 2005;353(8):839‐41. - PubMed
Antcliff 1999
-
- Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Seminars in Ophthalmology 1999;14(4):223‐32. - PubMed
Arevalo 2013
-
- Arevalo JF, Lasave AF, Wu L, Diaz‐Llopis M, Gallego‐Pinazo R, Alezzandrini AA, et al. Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: results of the Pan‐american Collaborative Retina Study Group at 24 months. Retina 2013;33(2):403‐13. - PubMed
ATC 1994
Banfi 2013
-
- Banfi R, Attanasio F, Palazzi N, Colombini S, Falai T, Cecchi M, et al. Bevacizumab versus ranibizumab: why are we not playing the joker?. International Journal of Clinical Pharmacy 2013;35(4):507‐9. - PubMed
Borm 2009
-
- Borm GF, Lemmers O, Fransen J, Donders R. The evidence provided by a single trial is less reliable than its statistical analysis suggests. Journal of Clinical Epidemiology 2009;62(7):711‐5. - PubMed
Brown 2004
-
- Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Archives of Ophthalmology 2004;122(3):330‐5. - PubMed
Browning 2008
Campochiaro 2010
-
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102‐12. - PubMed
CATT 2011
Chaimani 2013
Chong 2016
Ciulla 2003
-
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003;26(9):2653‐64. - PubMed
Ciulla 2004
-
- Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Current Opinions in Ophthalmology 2004;15(3):211‐20. - PubMed
Cunningham 2005
-
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747‐57. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29:932‐944. - PubMed
ETDRS 1985
-
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796‐806. - PubMed
EURETINA 2017
-
- Schmidt‐Erfurth U, Garcia‐Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017 Apr 20 [Epub ahead of print]. - PubMed
Ford 2012
Frank 2004
-
- Frank RN. Diabetic retinopathy. New England Journal of Medicine 2004;350(1):48‐58. - PubMed
Glanville 2006
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 6 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grover 2008
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. - PubMed
Haller 2010
-
- Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Archives of Ophthalmology 2010;128(3):289‐96. - PubMed
Heier 2016
-
- Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmology 2016;134(1):95‐9. - PubMed
Higgins 2011a
-
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2014
-
- Higgins JP, Giovane C, Chaimani A, Caldwell DM, Salanti G. Evaluating the quality of evidence from a network meta‐analysis. Value Health 2014;17:A324. - PubMed
Hussain 2015
-
- Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti‐VEGF treatments, adopting corticosteroid‐based treatments, and combination therapy. Expert Opinion on Biological Therapy 2016;16(3):365‐74. - PubMed
Jampol 2014
Jiang 2015
Klein 1984
-
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91(12):1464‐74. - PubMed
Korobelnik 2015
Kuppermann 2010
-
- Kuppermann BD, Chou C, Weinberg DV, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Archives of Ophthalmology 2010;128(5):642‐3. - PubMed
MEDCAC 2012
-
- Anti‐vascular endothelial growth factor treatment for diabetic macular edema. www.cms.gov/medicare‐coverage‐database/details/technology‐assessments‐de... (accessed 24 Sept 2012).
Moja 2014
Olson 2013
Ontario HTA 2009
OZDRY 2015
Pagliarini 2014
-
- Pagliarini S, Beatty S, Lipkova B, Perez‐Salvador Garcia E, Reynders S, et al. A 2‐year, phase IV, multicentre, observational study of ranibizumab 0.5 mg in patients with neovascular age‐related macular degeneration in routine clinical practice: The EPICOHORT study. Journal of Ophthalmology 2014;2014:Article ID 857148. [DOI: 10.1155/2014/857148] - DOI - PMC - PubMed
Patrao 2016
-
- Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real‐world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom national health service setting. American Journal of Ophthalmology 2016;172:51‐7. - PubMed
PLACID 2013
-
- Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120(9):1843‐51. - PubMed
Regnier 2014
Review Manager 5 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3:80‐97. - PubMed
Salanti 2014
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions.In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stewart 2016
-
- Stewart MW, Narayanan R, Gupta V, Rosenfeld PJ, Martin DF, Chakravarthy U. Counterfeit Avastin in India: Punish the criminals, not the patients. American Journal of Ophthalmology 2016;170:228‐31. - PubMed
Tranos 2004
-
- Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Survey of Ophthalmology 2004;49(5):470‐90. - PubMed
White 2015
-
- White IR. Network meta‐analysis. Stata Journal 2015;15:951‐85.
Yau 2012
Zhang 2016
References to other published versions of this review
Parravano 2008
Parravano 2009
Virgili 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

