Current and future applications of 3D printing in congenital cardiology and cardiac surgery
- PMID: 30325646
- PMCID: PMC6404827
- DOI: 10.1259/bjr.20180389
Current and future applications of 3D printing in congenital cardiology and cardiac surgery
Abstract
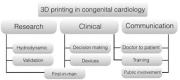
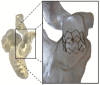
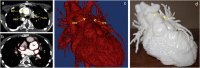
Three-dimensional (3D) printing technology in congenital cardiology and cardiac surgery has experienced a rapid development over the last decade. In presence of complex cardiac and extra-cardiac anatomies, the creation of a physical, patient-specific model is attractive to most clinicians. However, at the present time, there is still a lack of strong scientific evidence of the benefit of 3D models in clinical practice and only qualitative evaluation of the models has been used to investigate their clinical use. 3D models can be printed in rigid or flexible materials, and the original size can be augmented depending on the application the models are needed for. The most common applications of 3D models at present include procedural planning of complex surgical or interventional cases, in vitro simulation for research purposes, training and communication with patients and families. The aim of this pictorial review is to describe the basic principles of this technology and present its current and future applications.
Figures










References
-
- Garekar S, Bharati A, Chokhandre M, Mali S, Trivedi B, Changela VP, et al. . Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J Pediatr Congenit Heart Surg 2016; 7: 344–50. doi: 10.1177/2150135116645604 - DOI - PubMed
-
- Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, et al. . Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg 2017; 52: 1139–48. doi: 10.1093/ejcts/ezx208 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

