Safety, tolerability, and pharmacokinetics of AL-335 in healthy volunteers and hepatitis C virus-infected subjects
- PMID: 30325939
- PMCID: PMC6191080
- DOI: 10.1371/journal.pone.0204974
Safety, tolerability, and pharmacokinetics of AL-335 in healthy volunteers and hepatitis C virus-infected subjects
Abstract
Background: The nucleotide analog AL-335 is a pangenotypic hepatitis C virus (HCV) nonstructural protein (NS)5B inhibitor being evaluated as treatment for chronic HCV infection.
Methods: This three-part randomized, double-blind study evaluated the safety and pharmacokinetics of single and multiple ascending oral doses of AL-335. Healthy volunteers (HVs) received single doses of AL-335 (100-1,200 mg) or placebo in a fasted or fed (400 mg) state. Non-cirrhotic subjects (HCV genotype [GT]1-4) and GT1-infected subjects with Child Pugh A cirrhosis received multiple doses of AL-335 (400, 800, 1,200 mg) or placebo once daily (QD) for 7 days.
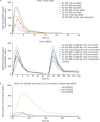
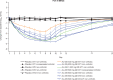
Results: Forty-eight HVs and 64 subjects with HCV GT1-4 were randomized and received treatment. AL-335 was well tolerated in HVs and HCV-infected subjects with/without cirrhosis. AL-335 was rapidly absorbed and converted to the metabolites ALS-022399 and ALS-022227. ALS-022227 exposure increased less than dose-proportionally and was unaffected by food, while AL-335 and ALS-022399 exposure increased with food by 85% and 50%, respectively, in HVs. Rapid and dose-dependent reductions in HCV-RNA were observed in GT1-infected subjects. In non-cirrhotic, GT1-4-infected subjects receiving AL-335 800 mg QD, potent antiviral activity was observed, regardless of genotype (mean maximum reductions in HCV-RNA of 4.0-4.8 log10 IU/mL). The same dose in GT1-infected cirrhotic subjects resulted in a 3.5 log10 IU/mL mean maximum reduction in HCV-RNA.
Conclusions: AL-335 was well tolerated when administered as single and multiple doses, with an acceptable pharmacokinetic profile. The drug also demonstrated potent antiviral activity in HCV GT1-4-infected subjects, including GT1-infected subjects with cirrhosis.
Trial registration: ClinicalTrials.gov NCT02339207.
Conflict of interest statement
The authors have read the journal's policy and the authors of this manuscript have the following competing interests: MWM and LB were employees of Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, at the time of the study. EB has been the Principal Investigator in clinical trials on HCV sponsored by Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, Atea Pharmaceuticals, and Janssen. TT has a project-based contractual relationship with ARENSIA. AS-C has been the Principal Investigator in clinical trials on HCV sponsored by AbbVie, Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, Boehringer Ingelheim, Janssen, and Merck Sharp & Dohme. LV is an employee of Janssen and a shareholder of Johnson & Johnson. BA and AP are employees of Biotrial. CW, TNK, JV, and NK are employees of Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, and are shareholders of Johnson & Johnson. SC, QZ, LMB, and JF were employees of Alios BioPharma Inc., part of the Janssen Pharmaceutical Companies, at the time of the study, and are shareholders of Johnson & Johnson. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- World Health Organization. Hepatitis C–Fact Sheet 164. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/
-
- American Association for the Study of Liver Diseases, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing and treating hepatitis C. 2017. Available from: https://www.hcvguidelines.org/ - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

