Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women
- PMID: 30325982
- PMCID: PMC6439556
- DOI: 10.1001/jamaoncol.2018.4270
Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women
Abstract
Importance: As cervical cancer screening transitions to primary human papillomavirus (HPV) testing, effective triage and management of HPV-positive women is critical to avoid unnecessary colposcopy referral and associated harms while maintaining high sensitivity for cervical precancer. Triage with p16/Ki-67 dual-stain (DS) testing has shown high sensitivity and specificity for detection of cervical precancers; however, longitudinal studies are needed to determine the long-term risk of precancer following a negative DS result.
Objective: To evaluate the longitudinal performance of p16/Ki-67 DS triage for detection of cervical precancer in HPV-positive women over 5 years of follow-up in the context of clinical management thresholds.
Design, setting, and participants: Prospective cohort study of HPV-positive women 30 years or older undergoing routine cervical cancer screening in 2012 with HPV and Papanicolaou (hereinafter "cytology") co-testing within the Kaiser Permanente Northern California health care system. Follow-up of medical records was conducted through 2017.
Exposures: All p16/Ki-67 DS testing was performed on residual SurePath material, and slides were evaluated for p16/Ki-67 positivity.
Main outcomes and measures: Histological end points were ascertained from the clinical database through 2017. We estimated 5-year cumulative risks of cervical intraepithelial neoplasia grades of 2 or worse (≥CIN2) or grades 3 or worse (≥CIN3) by baseline DS and cytology at yearly intervals using Logistic Weibull models. Risks were compared with clinical management thresholds for colposcopy referral and a 1-year return interval.
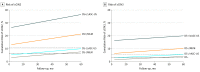
Results: Among the 1549 HPV-positive women in this study, the mean age at enrollment was 42.2 years, and the median follow-up time was 3.7 years (range, 0.2-5.4 years). Positive DS results were associated with significantly higher cumulative 5-year risks of ≥CIN2 compared with abnormal cytology (31.0%; 95% CI, 27.2%-35.3% vs 25.0%; 95% CI, 21.7%-28.7%; P = .03). Women with DS-negative findings had significantly lower 5-year risks of ≥CIN2 compared with women with normal cytology (8.5%; 95% CI, 6.5%-11.1% vs 12.3%; 95% CI, 9.8%-15.4%; P = .04). In DS-negative women, the risks of both ≥CIN2 and ≥CIN3 remained below the colposcopy referral threshold for all 5 years, crossing the 1-year return threshold at 3 years.
Conclusions and relevance: Triage with p16/Ki-67 DS provides better long-term risk stratification than cytology over 5 years. The low risk of cervical precancer in p16/Ki-67 DS-negative women permits safe extension of follow-up intervals for 3 years.
Conflict of interest statement
Figures

References
-
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175-204. doi: 10.1097/LGT.0b013e31824ca9d5 - DOI - PMC - PubMed
-
- Benevolo M, Allia E, Gustinucci D, et al. ; New Technologies for Cervical Cancer Screening 2 (NTCC2) Working Group . Interobserver reproducibility of cytologic p16INK4a /Ki-67 dual immunostaining in human papillomavirus-positive women. Cancer Cytopathol. 2017;125(3):212-220. doi: 10.1002/cncy.21800 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

